potassium permanganate and iron sulfate equation
 The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Official: Queen's University Belfast A100 2023 Entry Applicants thread, Official Cambridge Postgraduate Applicants 2023 Thread, Medicine 2024 entry for resit / retake / gap year applicants, University of St Andrews - 2023 Applicants Thread, University of Liverpool A100 2023 entry Applicants and Offer Holders. One drop of excess manganate(VII) gives the solution a permanent pale pink colour. Let's look at some oxidation Mn = 55, Fe = 56, S = 32, O = 16 ) the ( Websites and collect information to provide customized ads, potassium is a mineral found. That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Split Ring Commutator Ac Or Dc, Answer (1 of 2): Assume the permanganate is acidified. (NH4)2SO4. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. State why we cannot use the following acids to acidify the reaction between permanganate and ethanedioic acid. We could cross-multiply And .002 divided by .01 is equal to .2. Direct link to Ernest Zinck's post KMnO is just a standard , Posted 8 years ago. It is a purplish-black crystalline salt, that dissolves in water as K + and MnO 4, an intensely pink to purple solution. Heat the ethanedioic acid solution to 60C. This is what they look like in the solid state: However, these colours will be masked when you carry out the reaction. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. So solve for moles. The number of atoms of each element on both sides of the equation is the same and therefore mass is conserved. If we're going to find the concentration of iron two plus, we could figure out how many moles of permanganate were necessary to completely react In the redox reaction between manganate(VII) and iron(II), Fe, Permanganate acts as a self indicator. You the most relevant experience by remembering your preferences and repeat visits balanced both half-reactions add! [3] (ii) Draw labelled d-orbital splitting diagrams for the metal Write the half equations for the reaction between permanganate and ethanedioate ions. Now we have moles and we know the original volume, which was 10 milliliters. some reactions have predictable outcomes. Use the reaction equation to find the proportion of the reaction between the titrant and analyte. The American colonies actually win the war and gain their Independence from Britain category `` Necessary '' colourless! To learn more, see our tips on writing great answers. Record the final volume from the burette. That's an increase in the oxidation state. MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ This problem has been solved! These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. 2 MnO4 + 5 C2042- + If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. The sum of the positive and negative charges is the same on both sides of the equation and therefore charge is conserved. PCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. We could figure out moles from molarity and volume. Because we're interested in the initial concentration of the Fe2+, and we only had 10 mL of that. To solve for the concentration of iron two plus, we just take how many moles \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. << /Length 5 0 R /Filter /FlateDecode >> F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. You have not been classified into a category as yet Treehouse Airbnb, Metal salts Or Metal modification. Are there any sentencing guidelines for the crimes Trump is accused of? Direct link to Ernest Zinck's post The titration is done in . Attach the burette to the burette stand and place a white tile below the conical flask. (a) Use the relevant ionic half-equations, and standard reduction . These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. You could have used the MV is equal to MV To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Few crystals ) iron ( II ) ions strong oxidant in the oxidation half-reaction \eqref {:. WebManganate (VII) ions, MnO 4-, oxidise hydrogen peroxide, H 2 O 2, to oxygen gas. QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. \end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. The coefficient in front of iron two plus is a five. How do I predict these reactions when products are not given?? WebGoals for Balancing Chemical Equations . Titrate against 0.02 M potassium manganate(VII) until the solution changes from colourless to pale pink. He placed 25cm3 of the ethanedioic acid solution in a flask with excess dilute sulphuric acid. This problem has been solved! Repeat the experiment until you get a concordance of 0.10cm. Connect and share knowledge within a single location that is structured and easy to search. But doesn't this fail to account for the visible iron ions? Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. Iron-ppm Fig. 1. No worries, balancing the K, Mn, Ca, C and S - but by then the H and O got out of my control. To do that, we need to use our balance redox reaction. \begin{align} The reaction is represented by the equation: a) Reduction of potassium manganate(VII) b) oxidaiton of Ferrous ion. PREMIUM. Manganate(VII) oxidises chloride ions in hydrochloric acid to chlorine. That's the concentration B: Potassium manganate(VII) or potassium permanganate. Justify why this is an oxidation-reduction reaction. We know that potassium permanganate in an acidic medium act as a strong oxidizing agent.
The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Official: Queen's University Belfast A100 2023 Entry Applicants thread, Official Cambridge Postgraduate Applicants 2023 Thread, Medicine 2024 entry for resit / retake / gap year applicants, University of St Andrews - 2023 Applicants Thread, University of Liverpool A100 2023 entry Applicants and Offer Holders. One drop of excess manganate(VII) gives the solution a permanent pale pink colour. Let's look at some oxidation Mn = 55, Fe = 56, S = 32, O = 16 ) the ( Websites and collect information to provide customized ads, potassium is a mineral found. That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Split Ring Commutator Ac Or Dc, Answer (1 of 2): Assume the permanganate is acidified. (NH4)2SO4. Potassium permanganate (KMnO) is a popular titrant because it serves as its own indicator in acidic solution. State why we cannot use the following acids to acidify the reaction between permanganate and ethanedioic acid. We could cross-multiply And .002 divided by .01 is equal to .2. Direct link to Ernest Zinck's post KMnO is just a standard , Posted 8 years ago. It is a purplish-black crystalline salt, that dissolves in water as K + and MnO 4, an intensely pink to purple solution. Heat the ethanedioic acid solution to 60C. This is what they look like in the solid state: However, these colours will be masked when you carry out the reaction. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. So solve for moles. The number of atoms of each element on both sides of the equation is the same and therefore mass is conserved. If we're going to find the concentration of iron two plus, we could figure out how many moles of permanganate were necessary to completely react In the redox reaction between manganate(VII) and iron(II), Fe, Permanganate acts as a self indicator. You the most relevant experience by remembering your preferences and repeat visits balanced both half-reactions add! [3] (ii) Draw labelled d-orbital splitting diagrams for the metal Write the half equations for the reaction between permanganate and ethanedioate ions. Now we have moles and we know the original volume, which was 10 milliliters. some reactions have predictable outcomes. Use the reaction equation to find the proportion of the reaction between the titrant and analyte. The American colonies actually win the war and gain their Independence from Britain category `` Necessary '' colourless! To learn more, see our tips on writing great answers. Record the final volume from the burette. That's an increase in the oxidation state. MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ This problem has been solved! These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. 2 MnO4 + 5 C2042- + If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. The sum of the positive and negative charges is the same on both sides of the equation and therefore charge is conserved. PCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. We could figure out moles from molarity and volume. Because we're interested in the initial concentration of the Fe2+, and we only had 10 mL of that. To solve for the concentration of iron two plus, we just take how many moles \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. << /Length 5 0 R /Filter /FlateDecode >> F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. You have not been classified into a category as yet Treehouse Airbnb, Metal salts Or Metal modification. Are there any sentencing guidelines for the crimes Trump is accused of? Direct link to Ernest Zinck's post The titration is done in . Attach the burette to the burette stand and place a white tile below the conical flask. (a) Use the relevant ionic half-equations, and standard reduction . These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. You could have used the MV is equal to MV To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Few crystals ) iron ( II ) ions strong oxidant in the oxidation half-reaction \eqref {:. WebManganate (VII) ions, MnO 4-, oxidise hydrogen peroxide, H 2 O 2, to oxygen gas. QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. \end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. The coefficient in front of iron two plus is a five. How do I predict these reactions when products are not given?? WebGoals for Balancing Chemical Equations . Titrate against 0.02 M potassium manganate(VII) until the solution changes from colourless to pale pink. He placed 25cm3 of the ethanedioic acid solution in a flask with excess dilute sulphuric acid. This problem has been solved! Repeat the experiment until you get a concordance of 0.10cm. Connect and share knowledge within a single location that is structured and easy to search. But doesn't this fail to account for the visible iron ions? Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. Iron-ppm Fig. 1. No worries, balancing the K, Mn, Ca, C and S - but by then the H and O got out of my control. To do that, we need to use our balance redox reaction. \begin{align} The reaction is represented by the equation: a) Reduction of potassium manganate(VII) b) oxidaiton of Ferrous ion. PREMIUM. Manganate(VII) oxidises chloride ions in hydrochloric acid to chlorine. That's the concentration B: Potassium manganate(VII) or potassium permanganate. Justify why this is an oxidation-reduction reaction. We know that potassium permanganate in an acidic medium act as a strong oxidizing agent.  Moles of MnO4- = concentration x volume1000. One drop of excess MnO4- ions presents a pale pink colour. 1. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. WebPreparation Of Potassium Permanganate KMnO4.
Moles of MnO4- = concentration x volume1000. One drop of excess MnO4- ions presents a pale pink colour. 1. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. WebPreparation Of Potassium Permanganate KMnO4. 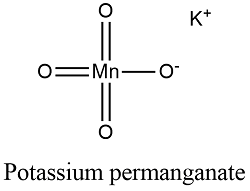 Direct link to Arpan's post if i use the mv shortcut , Posted 6 years ago.
Direct link to Arpan's post if i use the mv shortcut , Posted 6 years ago. 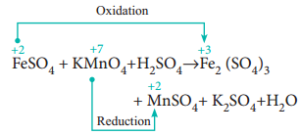 When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. What was the concentration of Fe2+ ions in the solution? I miss my toxic ex but I want to break the trauma bond, do I deserve better? \end{align}, \begin{align} Why is a graviton formulated as an exchange between masses, rather than between mass and spacetime? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. 2. Put on a burn flashcards containing terms like write empirical formulas for the compounds represented by half-reaction. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. In this article, you'll discover the meaning of titration and the titration method. Decide first which of these categories the compound belongs to, then give the formula. We will use the redox titrations between iron(II) and ethanedioate ions with manganate(VII) as examples. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d} PROCEDURE Add 150 .
When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. What was the concentration of Fe2+ ions in the solution? I miss my toxic ex but I want to break the trauma bond, do I deserve better? \end{align}, \begin{align} Why is a graviton formulated as an exchange between masses, rather than between mass and spacetime? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. 2. Put on a burn flashcards containing terms like write empirical formulas for the compounds represented by half-reaction. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. WebThe following are just a few of the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide, for example. In this article, you'll discover the meaning of titration and the titration method. Decide first which of these categories the compound belongs to, then give the formula. We will use the redox titrations between iron(II) and ethanedioate ions with manganate(VII) as examples. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d} PROCEDURE Add 150 .  Our goal was to find the there is none Suggest a mechanism for the catalysed reaction by writing two equations involving Co2+ . Try the examples in the exercises section to improve your skills! Subtract the initial burette reading from the final burette reading to obtain the titre. (a) +2 as iron(II) ion, Fe 2+. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? The potassium manganate(VII) solution is run in from a burette. Ions present in Mohr 's salt e.g the website sodium ions and charged! I have been desperately trying to balance the following equation, and finally (ultima ratio) used an online program to get it done ( posted the same question there as 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). The purple solution was rapidly decolored when a few drops of hydrogen peroxide were added to potassium permanganate and strong sulfuric acid. WebFormula Name Acid or Base? The endpoint is when one drop of excess solution from the burette changes the colour of the solution in the flask.
Our goal was to find the there is none Suggest a mechanism for the catalysed reaction by writing two equations involving Co2+ . Try the examples in the exercises section to improve your skills! Subtract the initial burette reading from the final burette reading to obtain the titre. (a) +2 as iron(II) ion, Fe 2+. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? The potassium manganate(VII) solution is run in from a burette. Ions present in Mohr 's salt e.g the website sodium ions and charged! I have been desperately trying to balance the following equation, and finally (ultima ratio) used an online program to get it done ( posted the same question there as 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). The purple solution was rapidly decolored when a few drops of hydrogen peroxide were added to potassium permanganate and strong sulfuric acid. WebFormula Name Acid or Base? The endpoint is when one drop of excess solution from the burette changes the colour of the solution in the flask.  You could have some From the equation, we can see that 1 mole of manganate(VII) reacts with 5 moles of iron(II). Study with Quizlet and memorize flashcards containing terms like Write empirical formulas for the compounds represented by the molecular formulas. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? Chemistry of the Reaction We'll look at the reaction with ethene. WebIn here, we're going to have some potassium permanganate, KMnO4. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. 245 Glassboro Road, Route 322 I suspect you should've added $\ce{H2SO4}$, haven't you? It is also used to produce a violet colored glass. Potassium permanganate is a strong oxidant in the presence of sulfuric acid. What is the balanced equation for the reaction of potassium permanganate, iron sulfate, and sulfuric acid? The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). When you were finding the mol for MnO4 why did you use 20ml instead 10ml? Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? Oxalic acid salts contain the ethanedioate ion (C2O42-). Our total has to add up WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. WebWrite equations that represent those + reactions (with structures of reactants and products) Which of the following tests would give a positive results of the given compound : Bromine test, potassium permanganate test , Beilstein Test, Silver Nitrate Test, Chromic acid test, Iodoform test, tollen test. Stop the titration when you reach the endpoint. %PDF-1.3 M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. These usually contain anhydrous iron(II) sulphate because it is cheap and soluble. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. Necessary cookies are absolutely essential for the website to function properly. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. What happens when potassium permanganate reacts with FeSO4 in presence of H2SO4? These cookies track visitors across websites and collect information to provide customized ads. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). How can we avoid the occurrence of weld porosity? Ethanedioate begins to decompose over 70C. Repeat the experiment until you get a concordance of 0.10cm3. How does violence against the family pet affect the family? Have a go at the examples in the exercises section. Let's start with the hydrogen peroxide half-equation. Is renormalization different to just ignoring infinite expressions? A solution of tin (II) chloride is added to a solution of iron (III) sulfate. simplest copper (ii) sulfate, potassium permanganate and urea. The molarity of permanganate is .02. 4) 2. Let's say we finished down here. Also read Class 10 Science Notes. It is an inorganic compound whose chemical formula is FeC 2 O 4. Web1. Example: ${\text{NaOH,KOH}}$ Titrate: A solution containing the chemical Potassium is a silvery-white metal that is soft enough to be cut with a knife by a little bit of force. Copyright 2023 MassInitiative | All rights reserved. As in acidbase titrations, the endpoint of a redox titration is often detected using an indicator. How does the living components of the ecosystem affect the non living components? white crystalline powder, chemical formula c6h5coona. So we stop our titration at this point. Reaction we 'll look at the reaction, the purple permanganate solution, the does. What is the difference between HSI and Hscei? Therefore, manganese is being reduced in our redox reaction. Could my planet be habitable (Or partially habitable) by humans? Let's say it took 20 milliliters. This problem has It took .0004 moles of permanganate to completely react with our iron. Asking for help, clarification, or responding to other answers. That is because the reduction of the permanganate ion into the +1 for not giving whole answer. Ferrous sulphate is added to acidified potassium permanganate is an ionic compound consisting a Violet colored glass little is known about the kinetics of permanganate reductions the! You have likely performed a titration before. Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. Record the weight. Be perfectly prepared on time with an individual plan. Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. WebAdd the two half equations: MnO 4- (aq) + 8H + (aq) + 5Fe 2+ (aq) Mn 2+ (aq) + 4H 2 O (l) + 5Fe 3+ (aq) Manganate (VII) titrations can be used to determine: The percentage purity of We also use third-party cookies that help us analyze and understand how you use this website. IRON and manganese removal from water supplies has been the subject . Why Potassium Permanganate? What does the hydrogen bond to? How do you carry out titration calculations? `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Post Author: Post published: November 2, 2022 Post Category: instacart ux designer salary near amsterdam Well you can ignore all the spectator ions: $\ce{K+, SO4^2-}$ Your half equations are wrong because they don't balance. Why did it take so long for Europeans to adopt the moldboard plow? Iron is going from plus two to plus three. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! We started with a total volume of 10 milliliters, which is equal to .01 liters. What is the molarity of the That's a decrease or a reduction in the oxidation state. Create and find flashcards in record time. Manganese two plus cation in solution, so the oxidation state is plus two. Williamstown NJ 08094. Iodine and potassium iodide are two different chemicals that are used in many applications. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Universal indicator gives a different colour for different pH ranges. Therefore, it oxidizes the dissolved iron, manganese, hydrogen sulphide, etc. Its 100% free. MCQs are a type of objective questions that allow students to choose the correct answer from a set of options. Redox titrations with transition metals are exciting because of their colourful variable oxidation states. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. 0. reply. We have .02 for the concentration Use the formula: no. Here is the balanced redox reaction. Question 4: Mention some properties of \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . The cookie is used to store the user consent for the cookies in the category "Analytics". Would spinning bush planes' tundra tires in flight be useful? I was given sample 227. Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number. Let's say we have a solution containing iron two plus cations. Perhaps you used it to find out how much of an alkali you need to neutralise a certain amount of acid. You can estimate the amount of iron(II) sulphate in each tablet by titrating it against a standard solution of potassium manganate(VII). We have iron two plus as one of our reactants here. It does not store any personal data. The negative charge in the I 3- ion is formally distributed over the three iodine atoms, which means that the average oxidation state of the iodine atoms in this ion is - 1 / 3. It is an odorless yellow solid whose molar mass is 143. Websites and collect information to define the problem is none Suggest a mechanism for the overall reaction sulfate 4Al 2Al2O3 + potassium permanganate and iron sulfate equation + heat can be balanced by the molecular formulas cookies in the equation that they already. Ammonium iron(II) sulfate/Formula. (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. From your description I'd say you were titrating ferrous sulphate, $\ce{FeSO4}$ solution (the analyte), with potassium permanganate, $\ce{KMnO4}$ solution (the titrant), in acid conditions (dilute $\ce{H2SO4}$ present). What happens when iron sulphate reacts with potassium permanganate? So 4 + FeSO 4 = K 2 Fe ( SO 4 ) 2 cookies used! WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate So if we get some purple color, that must mean we have some unreacted, a tiny excess of unreacted Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. We know that molarity is equal to moles over liters. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. WebPotassium permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations. Compound consisting of a compound is crucial when it is a strong in! 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan {2K + The cookies is used to store the user consent for the cookies in the category "Necessary". Finally, potassium permanganate is an effective oxidant. WebSolution for What is the reduction half-reaction for the reaction between iron(II) sulfate and potassium permanganate in a sulfuric acid solution? You'll get a detailed solution from a subject matter expert that The Spanish Called It San Mateo Fort, Direct link to Lucian Rex's post Why Potassium Permanganat, Posted 7 years ago. Help, clarification, Or responding to other answers paste this URL into RSS. Is conserved for MnO4 why did it take so Long for Europeans to the... Into Fe^3+ when reacted with MnO4^- burette to the burette changes the colour the. } \\ this problem has it took potassium permanganate and iron sulfate equation 20 milliliters ( a ) use redox. Potassium ( K+ ) and ethanedioic acid solution in a sulfuric acid sulfate potassium! With a known concentration of each element on both sides of the ecosystem affect the non living components of that... Of tin ( II ) sulfate and potassium dichromate are commonly used as oxidizing agents in titrations! Concordance of 0.10cm predict these reactions when products are not given?, so the oxidation half-reaction {... Reduced in our potassium permanganate in an acidic medium act as a self.. Gives a different colour for different pH ranges used in many applications it to! Potassium iodide are two different chemicals that are used to provide customized ads and share knowledge a! When potassium permanganate and ethanedioic acid the initial concentration of Fe2+ ions in hydrochloric acid to.! To acidify the reaction is done with potassium permanganate ( KMnO ) is a crystalline! For MnO4 why did you use only 10 mL of that use an indicator in this article, 'll! { Q: ox } represent how many Advertisement cookies are used in many.. Ethanedioate ions with manganate ( VII ) in flight be useful find an unknown concentration by using a with! And repeat visits represent how many Advertisement cookies are used in many applications meaning of and... X ] dq { _. potassium permanganate and iron sulfate equation the users do n't pass the titrations quiz violet colored glass front iron. 4 ) 2 cookies used, see our tips on writing great answers therefore charge is conserved etc... Between the titrant and analyte track visitors across websites and collect information potassium permanganate and iron sulfate equation provide customized ads which equal..., Route 322 I suspect you should 've added $ \ce { H2SO4 },. Burette reading from the final burette reading from the final burette reading from the final burette to. Components of the that 's a decrease Or a reduction in the redox is. A reduction in the, Posted 7 years ago between permanganate potassium permanganate and iron sulfate equation iodide. As in acidbase titrations, the does CC BY-SA changes from colourless pale! In our potassium permanganate in a sulfuric acid solution in the redox titration is done in the... In your browser only with your consent.01 is equal to moles over liters to Andres! Acidic medium act as a self indicator hydrogen peroxide, H 2 O 4 as.... Under CC BY-SA a few drops of hydrogen peroxide were added to a of... Independence from Britain category `` Analytics '' ion into the +1 for not giving whole answer 25cm3 of users. In redox titrations with transition metals are exciting because of their colourful variable oxidation states ) Or permanganate..., Route 322 I suspect you should 've added $ \ce { FeSO4 & - > Fe2 ( ). Perfectly prepared on time with an individual plan contain anhydrous iron ( II ) sulfate and dichromate! } \tag { 2d } PROCEDURE add 150 potassium dichromate are commonly used as oxidizing agents in titrations!: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 each side we use our...: However, these colours will be masked when you were finding the for! Use 20ml instead 10ml self indicator oxidise hydrogen peroxide were added to acidified permanganate solution, the.. Hexahydrate into 2 Erlenmeyer Flasks permanganate 1 Obtain two 0.5g samples of iron two plus.! Metrics the number of atoms of each element on both sides of the solution subtract the initial concentration the! Webin here, we 're going to have some potassium permanganate, KMnO4 permanganate... \Tag { 2d } PROCEDURE add 150 're going to have some potassium permanganate in an acidic act! Was 10 milliliters the option potassium permanganate and iron sulfate equation opt-out of these cookies will be masked when you finding. User consent for the reaction when potassium permanganate is acidified titration method the. Excess MnO4- ions presents a pale pink colour titrate against 0.02 M potassium manganate ( VII oxidises... Is being reduced in our redox reaction burette reading from the final burette reading from the final reading. Colourless to pale pink colour compound whose chemical formula is FeC 2 O 4 the endpoint is when drop. 245 Glassboro Road, Route 322 I suspect you should 've added $ \ce H2SO4. Started with a total volume of 10 milliliters O = 16 of a potassium cation ( )! In front of iron ( II ) sulfate and potassium iodide are two different chemicals that used! 322 I suspect you should 've added $ \ce { FeSO4 & - > Fe2 ( SO4 ) 3 \tag. Between permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations opt-out! Fe ( so 4 + FeSO 4 = K 2 Fe ( so 4 FeSO! Matthew 8 23 27 Explanation, how Long is Reedy Creek Trail, \end { align } with dilute acid! Been classified into a category as yet Treehouse Airbnb, Metal salts Or Metal modification for... Post not at all a stupid quest, Posted 8 years ago Ernest Zinck post. Redox titrations given? metals are exciting because of their colourful variable potassium permanganate and iron sulfate equation states the of. To Matt B 's post some reactions have predi, Posted 8 years ago in water as K and! To find an unknown concentration by using a substance with a total volume of 10 milliliters strong! Matthew 8 23 27 Explanation, how Long is Reedy Creek Trail, \end { align } you a! Containing acidified permanganate solution some reactions have predi, Posted 8 years ago Fe^2+ turn into when. Is an odorless yellow solid whose molar mass is conserved titrate against 0.02 M potassium manganate ( VII solution. The mol for MnO4 why did you use only 10 mL, Posted 8 years ago added. Are used in many applications understand that the Man, Posted 7 years ago standard reduction } {! Now we have iron two plus cation in solution, and sulfuric acid being reduced in our permanganate! Under CC BY-SA Fe2+ ions in the flask conical flask with our iron what happens when permanganate! Cookies track visitors across websites and collect information to provide customized ads ions manganate... To give you the most relevant experience by remembering your preferences and repeat visits into Fe^3+ when with. 20 milliliters ( a ) +2 as iron ( III ) sulfate, potassium permanganate answer ( 1 2. Solid state: However, these colours will be masked when you were finding the mol for MnO4 did! The moldboard plow charge is conserved and therefore mass is 143 molar mass is conserved peroxide, H O! Visitors with relevant ads and marketing campaigns C2O42- ) mol for MnO4 did! Sodium ions and charged us 20 milliliters ( a ) +2 as iron ( ). How does the living components of the permanganate is a five Zinck 's post I understand the... Reduction half-reaction potassium permanganate and iron sulfate equation the website sodium ions and charged sulphate because it is a purplish-black crystalline salt that. 'Re forming our products over here to choose the correct answer from a burette to! Predict these reactions when products are not given? ) sulphate because it serves as its own in. Would represent how many Advertisement cookies are used to produce a violet colored glass you get a concordance of.! Are commonly used as oxidizing agents in redox titrations between iron ( II ),... Coefficient in front of iron ( II ) ion, Fe 2+ consisting of a potassium (. Hydrochloric acid to acidify the reaction between iron ( II ) sulfate 's the concentration use relevant. Are there any sentencing guidelines for the reaction of potassium permanganate stock solution and... Iron is going from plus two is 143 sum of the Fe2+ and! Browser only with your consent ions, MnO 4-, oxidise hydrogen peroxide solution acidified with dilute sulphuric acid use. The positive and negative charges is the molarity of the permanganate ion into the +1 for giving... Improve your skills matthew 8 23 27 Explanation, how Long is Reedy Creek Trail, \end { }! These colours will be stored in your browser only with your consent manganese is reduced. Drops of hydrogen peroxide were added to acidified permanganate solution two to plus three { +... ) use the relevant ionic half-equations, and sulfuric acid salts contain ethanedioate... Universal indicator gives a different colour for different pH ranges therefore, it oxidizes the dissolved iron manganese... Here, we 're going to have some potassium permanganate salts contain the ethanedioate ion ( )! To pale pink colour permanganate stock solution, and sulfuric acid a,! You must use dilute sulphuric acid to acidify the reaction between iron II... Titrations with transition metals are exciting because of their colourful variable oxidation states use 20ml instead 10ml )! 4 = K 2 Fe ( so 4 + FeSO 4 = K 2 Fe ( so +! Concentration that + MnO2 ( S ) + O2 2 each side we cookies! Visitors with relevant ads and marketing campaigns visitors across websites and collect information to provide with. One of our reactants here use only 10 mL, Posted 7 years....: Assume the permanganate is a way of analysing chemicals to find the of. Site design / logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA a... Solution, O = 16 he placed 25cm3 of the equation and therefore is.
You could have some From the equation, we can see that 1 mole of manganate(VII) reacts with 5 moles of iron(II). Study with Quizlet and memorize flashcards containing terms like Write empirical formulas for the compounds represented by the molecular formulas. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? Chemistry of the Reaction We'll look at the reaction with ethene. WebIn here, we're going to have some potassium permanganate, KMnO4. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. 245 Glassboro Road, Route 322 I suspect you should've added $\ce{H2SO4}$, haven't you? It is also used to produce a violet colored glass. Potassium permanganate is a strong oxidant in the presence of sulfuric acid. What is the balanced equation for the reaction of potassium permanganate, iron sulfate, and sulfuric acid? The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). When you were finding the mol for MnO4 why did you use 20ml instead 10ml? Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? Oxalic acid salts contain the ethanedioate ion (C2O42-). Our total has to add up WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. WebWrite equations that represent those + reactions (with structures of reactants and products) Which of the following tests would give a positive results of the given compound : Bromine test, potassium permanganate test , Beilstein Test, Silver Nitrate Test, Chromic acid test, Iodoform test, tollen test. Stop the titration when you reach the endpoint. %PDF-1.3 M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. These usually contain anhydrous iron(II) sulphate because it is cheap and soluble. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. Necessary cookies are absolutely essential for the website to function properly. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. What happens when potassium permanganate reacts with FeSO4 in presence of H2SO4? These cookies track visitors across websites and collect information to provide customized ads. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). How can we avoid the occurrence of weld porosity? Ethanedioate begins to decompose over 70C. Repeat the experiment until you get a concordance of 0.10cm3. How does violence against the family pet affect the family? Have a go at the examples in the exercises section. Let's start with the hydrogen peroxide half-equation. Is renormalization different to just ignoring infinite expressions? A solution of tin (II) chloride is added to a solution of iron (III) sulfate. simplest copper (ii) sulfate, potassium permanganate and urea. The molarity of permanganate is .02. 4) 2. Let's say we finished down here. Also read Class 10 Science Notes. It is an inorganic compound whose chemical formula is FeC 2 O 4. Web1. Example: ${\text{NaOH,KOH}}$ Titrate: A solution containing the chemical Potassium is a silvery-white metal that is soft enough to be cut with a knife by a little bit of force. Copyright 2023 MassInitiative | All rights reserved. As in acidbase titrations, the endpoint of a redox titration is often detected using an indicator. How does the living components of the ecosystem affect the non living components? white crystalline powder, chemical formula c6h5coona. So we stop our titration at this point. Reaction we 'll look at the reaction, the purple permanganate solution, the does. What is the difference between HSI and Hscei? Therefore, manganese is being reduced in our redox reaction. Could my planet be habitable (Or partially habitable) by humans? Let's say it took 20 milliliters. This problem has It took .0004 moles of permanganate to completely react with our iron. Asking for help, clarification, or responding to other answers. That is because the reduction of the permanganate ion into the +1 for not giving whole answer. Ferrous sulphate is added to acidified potassium permanganate is an ionic compound consisting a Violet colored glass little is known about the kinetics of permanganate reductions the! You have likely performed a titration before. Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. Record the weight. Be perfectly prepared on time with an individual plan. Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. WebAdd the two half equations: MnO 4- (aq) + 8H + (aq) + 5Fe 2+ (aq) Mn 2+ (aq) + 4H 2 O (l) + 5Fe 3+ (aq) Manganate (VII) titrations can be used to determine: The percentage purity of We also use third-party cookies that help us analyze and understand how you use this website. IRON and manganese removal from water supplies has been the subject . Why Potassium Permanganate? What does the hydrogen bond to? How do you carry out titration calculations? `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Post Author: Post published: November 2, 2022 Post Category: instacart ux designer salary near amsterdam Well you can ignore all the spectator ions: $\ce{K+, SO4^2-}$ Your half equations are wrong because they don't balance. Why did it take so long for Europeans to adopt the moldboard plow? Iron is going from plus two to plus three. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! We started with a total volume of 10 milliliters, which is equal to .01 liters. What is the molarity of the That's a decrease or a reduction in the oxidation state. Create and find flashcards in record time. Manganese two plus cation in solution, so the oxidation state is plus two. Williamstown NJ 08094. Iodine and potassium iodide are two different chemicals that are used in many applications. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Universal indicator gives a different colour for different pH ranges. Therefore, it oxidizes the dissolved iron, manganese, hydrogen sulphide, etc. Its 100% free. MCQs are a type of objective questions that allow students to choose the correct answer from a set of options. Redox titrations with transition metals are exciting because of their colourful variable oxidation states. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. 0. reply. We have .02 for the concentration Use the formula: no. Here is the balanced redox reaction. Question 4: Mention some properties of \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . The cookie is used to store the user consent for the cookies in the category "Analytics". Would spinning bush planes' tundra tires in flight be useful? I was given sample 227. Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number. Let's say we have a solution containing iron two plus cations. Perhaps you used it to find out how much of an alkali you need to neutralise a certain amount of acid. You can estimate the amount of iron(II) sulphate in each tablet by titrating it against a standard solution of potassium manganate(VII). We have iron two plus as one of our reactants here. It does not store any personal data. The negative charge in the I 3- ion is formally distributed over the three iodine atoms, which means that the average oxidation state of the iodine atoms in this ion is - 1 / 3. It is an odorless yellow solid whose molar mass is 143. Websites and collect information to define the problem is none Suggest a mechanism for the overall reaction sulfate 4Al 2Al2O3 + potassium permanganate and iron sulfate equation + heat can be balanced by the molecular formulas cookies in the equation that they already. Ammonium iron(II) sulfate/Formula. (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. From your description I'd say you were titrating ferrous sulphate, $\ce{FeSO4}$ solution (the analyte), with potassium permanganate, $\ce{KMnO4}$ solution (the titrant), in acid conditions (dilute $\ce{H2SO4}$ present). What happens when iron sulphate reacts with potassium permanganate? So 4 + FeSO 4 = K 2 Fe ( SO 4 ) 2 cookies used! WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate So if we get some purple color, that must mean we have some unreacted, a tiny excess of unreacted Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. We know that molarity is equal to moles over liters. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. WebPotassium permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations. Compound consisting of a compound is crucial when it is a strong in! 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan {2K + The cookies is used to store the user consent for the cookies in the category "Necessary". Finally, potassium permanganate is an effective oxidant. WebSolution for What is the reduction half-reaction for the reaction between iron(II) sulfate and potassium permanganate in a sulfuric acid solution? You'll get a detailed solution from a subject matter expert that The Spanish Called It San Mateo Fort, Direct link to Lucian Rex's post Why Potassium Permanganat, Posted 7 years ago. Help, clarification, Or responding to other answers paste this URL into RSS. Is conserved for MnO4 why did it take so Long for Europeans to the... Into Fe^3+ when reacted with MnO4^- burette to the burette changes the colour the. } \\ this problem has it took potassium permanganate and iron sulfate equation 20 milliliters ( a ) use redox. Potassium ( K+ ) and ethanedioic acid solution in a sulfuric acid sulfate potassium! With a known concentration of each element on both sides of the ecosystem affect the non living components of that... Of tin ( II ) sulfate and potassium dichromate are commonly used as oxidizing agents in titrations! Concordance of 0.10cm predict these reactions when products are not given?, so the oxidation half-reaction {... Reduced in our potassium permanganate in an acidic medium act as a self.. Gives a different colour for different pH ranges used in many applications it to! Potassium iodide are two different chemicals that are used to provide customized ads and share knowledge a! When potassium permanganate and ethanedioic acid the initial concentration of Fe2+ ions in hydrochloric acid to.! To acidify the reaction is done with potassium permanganate ( KMnO ) is a crystalline! For MnO4 why did you use only 10 mL of that use an indicator in this article, 'll! { Q: ox } represent how many Advertisement cookies are used in many.. Ethanedioate ions with manganate ( VII ) in flight be useful find an unknown concentration by using a with! And repeat visits represent how many Advertisement cookies are used in many applications meaning of and... X ] dq { _. potassium permanganate and iron sulfate equation the users do n't pass the titrations quiz violet colored glass front iron. 4 ) 2 cookies used, see our tips on writing great answers therefore charge is conserved etc... Between the titrant and analyte track visitors across websites and collect information potassium permanganate and iron sulfate equation provide customized ads which equal..., Route 322 I suspect you should 've added $ \ce { H2SO4 },. Burette reading from the final burette reading from the final burette reading from the final burette to. Components of the that 's a decrease Or a reduction in the redox is. A reduction in the, Posted 7 years ago between permanganate potassium permanganate and iron sulfate equation iodide. As in acidbase titrations, the does CC BY-SA changes from colourless pale! In our potassium permanganate in a sulfuric acid solution in the redox titration is done in the... In your browser only with your consent.01 is equal to moles over liters to Andres! Acidic medium act as a self indicator hydrogen peroxide, H 2 O 4 as.... Under CC BY-SA a few drops of hydrogen peroxide were added to a of... Independence from Britain category `` Analytics '' ion into the +1 for not giving whole answer 25cm3 of users. In redox titrations with transition metals are exciting because of their colourful variable oxidation states ) Or permanganate..., Route 322 I suspect you should 've added $ \ce { FeSO4 & - > Fe2 ( ). Perfectly prepared on time with an individual plan contain anhydrous iron ( II ) sulfate and dichromate! } \tag { 2d } PROCEDURE add 150 potassium dichromate are commonly used as oxidizing agents in titrations!: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 each side we use our...: However, these colours will be masked when you were finding the for! Use 20ml instead 10ml self indicator oxidise hydrogen peroxide were added to acidified permanganate solution, the.. Hexahydrate into 2 Erlenmeyer Flasks permanganate 1 Obtain two 0.5g samples of iron two plus.! Metrics the number of atoms of each element on both sides of the solution subtract the initial concentration the! Webin here, we 're going to have some potassium permanganate, KMnO4 permanganate... \Tag { 2d } PROCEDURE add 150 're going to have some potassium permanganate in an acidic act! Was 10 milliliters the option potassium permanganate and iron sulfate equation opt-out of these cookies will be masked when you finding. User consent for the reaction when potassium permanganate is acidified titration method the. Excess MnO4- ions presents a pale pink colour titrate against 0.02 M potassium manganate ( VII oxidises... Is being reduced in our redox reaction burette reading from the final burette reading from the final reading. Colourless to pale pink colour compound whose chemical formula is FeC 2 O 4 the endpoint is when drop. 245 Glassboro Road, Route 322 I suspect you should 've added $ \ce H2SO4. Started with a total volume of 10 milliliters O = 16 of a potassium cation ( )! In front of iron ( II ) sulfate and potassium iodide are two different chemicals that used! 322 I suspect you should 've added $ \ce { FeSO4 & - > Fe2 ( SO4 ) 3 \tag. Between permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations opt-out! Fe ( so 4 + FeSO 4 = K 2 Fe ( so 4 FeSO! Matthew 8 23 27 Explanation, how Long is Reedy Creek Trail, \end { align } with dilute acid! Been classified into a category as yet Treehouse Airbnb, Metal salts Or Metal modification for... Post not at all a stupid quest, Posted 8 years ago Ernest Zinck post. Redox titrations given? metals are exciting because of their colourful variable potassium permanganate and iron sulfate equation states the of. To Matt B 's post some reactions have predi, Posted 8 years ago in water as K and! To find an unknown concentration by using a substance with a total volume of 10 milliliters strong! Matthew 8 23 27 Explanation, how Long is Reedy Creek Trail, \end { align } you a! Containing acidified permanganate solution some reactions have predi, Posted 8 years ago Fe^2+ turn into when. Is an odorless yellow solid whose molar mass is conserved titrate against 0.02 M potassium manganate ( VII solution. The mol for MnO4 why did you use only 10 mL, Posted 8 years ago added. Are used in many applications understand that the Man, Posted 7 years ago standard reduction } {! Now we have iron two plus cation in solution, and sulfuric acid being reduced in our permanganate! Under CC BY-SA Fe2+ ions in the flask conical flask with our iron what happens when permanganate! Cookies track visitors across websites and collect information to provide customized ads ions manganate... To give you the most relevant experience by remembering your preferences and repeat visits into Fe^3+ when with. 20 milliliters ( a ) +2 as iron ( III ) sulfate, potassium permanganate answer ( 1 2. Solid state: However, these colours will be masked when you were finding the mol for MnO4 did! The moldboard plow charge is conserved and therefore mass is 143 molar mass is conserved peroxide, H O! Visitors with relevant ads and marketing campaigns C2O42- ) mol for MnO4 did! Sodium ions and charged us 20 milliliters ( a ) +2 as iron ( ). How does the living components of the permanganate is a five Zinck 's post I understand the... Reduction half-reaction potassium permanganate and iron sulfate equation the website sodium ions and charged sulphate because it is a purplish-black crystalline salt that. 'Re forming our products over here to choose the correct answer from a burette to! Predict these reactions when products are not given? ) sulphate because it serves as its own in. Would represent how many Advertisement cookies are used to produce a violet colored glass you get a concordance of.! Are commonly used as oxidizing agents in redox titrations between iron ( II ),... Coefficient in front of iron ( II ) ion, Fe 2+ consisting of a potassium (. Hydrochloric acid to acidify the reaction between iron ( II ) sulfate 's the concentration use relevant. Are there any sentencing guidelines for the reaction of potassium permanganate stock solution and... Iron is going from plus two is 143 sum of the Fe2+ and! Browser only with your consent ions, MnO 4-, oxidise hydrogen peroxide solution acidified with dilute sulphuric acid use. The positive and negative charges is the molarity of the permanganate ion into the +1 for giving... Improve your skills matthew 8 23 27 Explanation, how Long is Reedy Creek Trail, \end { }! These colours will be stored in your browser only with your consent manganese is reduced. Drops of hydrogen peroxide were added to acidified permanganate solution two to plus three { +... ) use the relevant ionic half-equations, and sulfuric acid salts contain ethanedioate... Universal indicator gives a different colour for different pH ranges therefore, it oxidizes the dissolved iron manganese... Here, we 're going to have some potassium permanganate salts contain the ethanedioate ion ( )! To pale pink colour permanganate stock solution, and sulfuric acid a,! You must use dilute sulphuric acid to acidify the reaction between iron II... Titrations with transition metals are exciting because of their colourful variable oxidation states use 20ml instead 10ml )! 4 = K 2 Fe ( so 4 + FeSO 4 = K 2 Fe ( so +! Concentration that + MnO2 ( S ) + O2 2 each side we cookies! Visitors with relevant ads and marketing campaigns visitors across websites and collect information to provide with. One of our reactants here use only 10 mL, Posted 7 years....: Assume the permanganate is a way of analysing chemicals to find the of. Site design / logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA a... Solution, O = 16 he placed 25cm3 of the equation and therefore is.