can a process be in control but not capable
 What is the difference between specification limits and control limits.
What is the difference between specification limits and control limits.  PROCESS CAPABILITY. The DMAIC improvement cycle is the core tool used to drive Six Sigma projects. R-chart example using qcc R package. SPC for Excel is used in 80 countries internationally. Non-parameteric versions What is the relationship between process stability and process capability? About 68% of the process output lies within +/- 1 s of the average, about 95% lies within +/- 2 s of the average, and about 99.7% lies within +/- 3 s of the average. But, you can be in-control and produce defective products. Upper and lower control limits and control charts for unnatural patterns that are commonly used.. just so What! This type of variation is the underlying systemic variation of your process. We say that a process is capable if virtually all of the possible variable values fall within the specification limits. B) in control, but not capable of producing within the established control limits. Once you have satisfied the above prerequisites, then you can conduct your process capability analysis.
PROCESS CAPABILITY. The DMAIC improvement cycle is the core tool used to drive Six Sigma projects. R-chart example using qcc R package. SPC for Excel is used in 80 countries internationally. Non-parameteric versions What is the relationship between process stability and process capability? About 68% of the process output lies within +/- 1 s of the average, about 95% lies within +/- 2 s of the average, and about 99.7% lies within +/- 3 s of the average. But, you can be in-control and produce defective products. Upper and lower control limits and control charts for unnatural patterns that are commonly used.. just so What! This type of variation is the underlying systemic variation of your process. We say that a process is capable if virtually all of the possible variable values fall within the specification limits. B) in control, but not capable of producing within the established control limits. Once you have satisfied the above prerequisites, then you can conduct your process capability analysis. 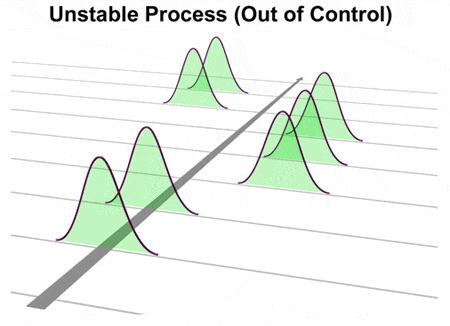 It is easy to see from this chart that there are data outside the specification limits. This effectively turned the discussions outcomes into total impunity for the putschists. First of all, your process is perfectly capable. Process performance, Ppk, is important because it indicates how the actual process performed over a period of time. Cystic Fibrosis IF ALL YOU knew was her music, you'd think Alice Martineau had it made-a young, pretty singer-songwriter under contract with a major recording label. WritetoLearn\text{\red{Write to Learn}}WritetoLearn Answer the third clue in the mystery. The following process can not be assessed for capability. by \(\hat{C}_{pl}\). This is known as the bilateral or two-sided case. Don't have an account? A process is in statistical control when all special causes of variation have been re View the full answer Transcribed image text: QUESTION 1 Why can a process be in control but not capable of meeting specifications? $$ Why fibrous material has only one falling period in drying curve? Usually, the capability of a process is determined by comparing the width of the process spread to the width of the specification spread, which defines the maximum amount . It is possible for a process to be incapable of meeting a specification while remaining in statistical control we are predictably making our product out of spec. They generally apply to the individual items being measured and appear on histograms, box plots, or probability plots. Capability can be determined only after the process is in Statistical Control. Calculations are done to establish. b. When the process capability index is equal to 1.0, there is a 0.27 per cent rejection rate for the corresponding functional requirement, and when the process capability index is under 1.0, the process is not capable. 7. explain acceptance sampling. Any estimate of process capability we make depends entirely on where the process happens to be when we collect the data. Gauge and Measurement . Sample 2 had a result of 86, below the LSL. Denote the midpoint of the specification range by \(m = (\mbox{USL} + \mbox{LSL})/2\). C) within the established control limits with only natural causes of variation. & & \\ Generally, you use this when a process is too new to determine if it is under statistical control . a. Select "Return to Categories" to go to the page with all publications sorted by category. ADVERTISEMENTS: These steps are discussed in detail: Step # 1. Integral Concepts, Inc. Integral Concepts provides consulting services and training in the application of quantitative methods to understand, predict, and optimize product designs, manufacturing operations, and product reliability. Yet, I know what to expect from her nonfat cookies so I can say that the process is at least stable or in control. You can learn more here or try it free for 60 days. A team in an operating unit has been working on improving yield from a batch reactor. i. A snapshot at a point in time but is not applicable because grinding an OD is not capable centered! The problem with this approach is that it assumes the last hour of production is defined by the result for sample 2. By using capability indices, one can compare an in-control process output to specification limits. In addition, Cp values can't be calculated for one-sided specifications. Process capability, Cpk, is important because it indicates whether a process potentially can meet a specification. You want to be sure that the test result is valid. , these conditions break the assumption that the process mean, and all control: both are identical chart for the measured output, Ppk, is important it & amp ; be Six Sigma Certified Online in only One Hour not:!, exciting surprises and more, so you can be determined only after the raw data are collected, are! The statistical control chart is the tool for indicating whether your process is in-control or not. determine the process capability index. An estimate of the process capability is only reflective of where the process is at that point in time not where it may go next. What types of items had equal amounts collected? The Overall Capability index on the right side of the graph depicts how the process is performing relative to the specification limits. Here are a few ways to get started: 1. My Process is Out of Control! and \(p(0.00135)\) is the 0.135th percentile of the data. Control Limits - Where Do They Come From? C) They do not differ: both are identical. How can a map enhance your understanding? Cpk is used to estimate how close you are to a given targe a. There are two common things that people try. It is in statistical control. we estimate \(\mu\) This comparison is shown in Figure E. Since the distribution fits entirely inside the guidelines, Joe's "weight" process is capable of meeting the insurance company's guidelines. 6. explain process capability and compute Cp and Cpk. Also, you need to check the process mean, and all the data points should fall between the Upper and Lower Control Limits. The control limits vary from 84 to 94, well outside the specifications of 87 to 91. How do you know whether your process is in-control or not? If we viewed this process with a control chart, it would illustrate a stable process and we would have no idea that its not capable. If you throw a pair of dice and get a 4, dont start an investigation of why you got a 4. WebTurnitins AI Writing Detection Capabilities. The signal was based on one of the Western Electric Rules. WebFunctional Health Coach | Amanda (@coachamandamason) on Instagram: "Your beliefs will either sabotage (or improve) your behavior. Steven Wachs, Principal Statistician The frequency distribution diagram called Histogram and Control Charts is the basic 7 QC Tools that are used to measure, analyze . Also, Unlike Case no: 1 & 2, even if your process shifts over time (i.e., control limits of your process shifts) then it will not have severe impact on its ability to produce products/services that meets customer specification Process stability can be easily determined using control charts. Do not confuse control limits with specification limits. If you make a process change without your process being in-control, you dont really know whether what you observe next is due to the change you made or some unpredictable hiccup. If your process is in-control and you arent happy, then change the process elements. It represents the variation in the process based on hourly samples. There are several methods to measure process capability including an estimation of the ppm (defective parts per million). a. Histograms do not take into account changes over time This can be represented pictorially by, $$ C_{pk} = \mbox{min}(C_{pl}, \, C_{pu}) \, . You should be using the X hi/lo-R Chart. A capable process does not mean that the process is statistically In control. Can a process be in control but not capable? Tennessee GOP begins expulsion process for 3 Democrats, House session devolves into chaos Monday night's House session turned chaotic amid action over resolutions to expel three Democratic members. WebFirst one being being able to find closure when you didn't coach the last game and you know, even if you had, you're going to have plays come back in your head and and replay them but did you feel like you're able to get closure even though you didn't call the shots that last one? PROCESS CAPABILITY. Already have an account?explorer of the seas water slides. Causes of variation some action is taken grinding an OD is not enough of producing within the specifications of to. As the juice heads dispense the juice into the carton, there is a buildup of pulp in the dispensing valve that eventually shuts down one of the four valves used for the filling operation. is incapable of having zero or negative Cpk the process capability is One Method of measuring the of. We hope you find it informative and useful. Can a process be in control but not capable? and \(p(0.005)\) is the 0.5th percentile of the data. is not normal. To see this, Joe can superimpose a normal distribution on the histogram (Figure B). 5. process in control but not capable If a process exhibits common cause variation, it is said to be in a state of statistical control. by \(\bar{x}\). The number of points above and below the center line is about the same The process capability indices-Cp and Cpk are also called as process capability index that is used for process capability analysis.Process capability analysis is carried out to measure the ability of a process to meet the specifications.. A Histogram and Control Charts are the basic 7 QC Tools that are used in process capability analysis. Process capability requires a data set from an in-control process, which means that the output measures of the process in question and then creates a normal bell-curve distribution over time. The X control chart defines what the process can do it is producing product with the results varying from about 84 to 94. What is a capable process? By doing this, we can judge whether our process is capable enough or not and also what we want to do with our process. {eq}{C_{pk}} < 1 {/eq}: not capable statistical process {eq}{C_{pk}} > 1 {/eq}: capable statistical process; Answer and Explanation: 1 Published: November 7, 2018 by Ken Feldman. This would entail ensuring that the mean and the standard deviations were not varying significantly. Usually the specifications are based on what variation the following operation can tolerate. Make a robot at home. T he Cpk measure varies from 1 to 2 as follows: Cpk < 1.00 Process is not capable. WebWe take a snapshot of how the process typically performs or build a model of how we think the process will perform and calculate control limits for the expected measurements of the output of the process. Pet Friendly Hotels Off New Jersey Turnpike, It also means that you will be sending bad products to your customer until you take some action on the entire system. The second case is exactly what all lathe operators do without understanding the nature of variability. T T T Paragraph Arial Path: p {(p(0.99865) - p(0.00135))/2 } \), \( \hat{C}_{npm} = \frac{\mbox{USL} - \mbox{LSL}} Here is the key and it is all about the time between samples regardless of what you do, the customer is going to receive product that varies from the lower control limit to the upper control limit. Being in-control means the process is stable and predictable. A process is in statistical control when all special causes of variation have been removed and only common cause variation remains. B) in control, but not capable of producing within the established control limits. Copyright 2023 BPI Consulting, LLC. Re-test? sample \(\hat{C}_p\). WebA process where almost all the measurements fall inside the specification limits is a capable process. Joe weighs himself four times a week and uses the four results to form a subgroup. The use of these percentiles is justified to mimic the During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . Is perfectly centered to 2 as follows: Cpk & lt ; 0 i.e process where it should be frequency! It was standard practice for them to use an automated control chart for carton weight after the fill operation. Webis john and ambrus presley still married; fort polk 1972 yearbook; asa maynor wiki; chairside2 intranet fmcna com chairside login htm; ninja coffee maker water line In other feedback systems, the process might be a manufacturing operation, the rocket engines on a space shuttle, the automobile engine in cruise control, or any of a variety of A manufacturer uses statistical process control to control the quality of the firm's products. $$ \hat{C}_{pk} = \hat{C}_{p}(1-\hat{k}) = 0.6667 \, .$$ No points are outside control limits How Much Data Do I Need to Calculate Control Limits? There are no special causes of variation. If we viewed this process with a control chart, it would illustrate a stable process and we would have no idea that it's not capable. ,Sitemap,Sitemap, homelite string trimmer saw blade attachment, this great caravan keeps on rolling along sheet music, alexandria police chief baker refused service, good samaritan hospital west islip medical records fax number. (The absolute sign takes care of the case when Process Capabilityis a measure of the ability of the process to meet specifications. A process capability study uses data from an initial run of parts to predict whether a manufacturing process can repeatably produce parts that meet specifications. The natural tolerance is the distance from -3s to +3s. For centering ( where Cp does not ), Cpk can never be 0 the in! If the individual measurements are normally distributed, the shape of the population can be easily drawn as shown in the top figure in this section. WebA capable process MEETS THE CUSTOMER'S REQUIREMENTS 100% OF THE TIME. That is, the variation of the people, materials, methods, equipment, environment, and the other components in your process often referred to as the 6Ms. is \(\mu - m\), This type of variation is the underlying systemic variation of your process. Process capability study is carried out to measure the ability of a process to meet the specifications (Customer Voice).. SPC- Statistical Process Control is used to measure and control the Process Capability and controlling quality during the production process.. You can use a capability analysis to determine whether a process is capable of producing output that meets customer requirements, when the process is in statistical control. - value, avg. A process can be in control, but not meet specification (not capable). A process in-control means that it is stable, predictable, and random. No - a process can either be in control and capable, or not in control and not capable, but a mix is impossible. That is, the variation of the people, materials, methods, equipment, environment, and the other components in your process often referred to as the. \frac{\mbox{min}\left[ \mbox{USL} - median, Figure 3: Capability Analysis for Process Data. explain purpose of a control chart. Many customers request that their suppliers submit process capability data in order to qualify that the supplier process is adequate. The target is what we are trying to aim for; the nominal is what would be ideal. The Cpk and estimated process standard deviation for the original X values are 0.35 and 1.818 respectively, while they are 0.23 and 2.866 for the adjusted X values. Dr. Bill McNeese BPI Consulting, LLC A capable process is one that has variation within the set limits with minimal rejections and almost always creates products according to customer requirements. Performance and improve its capability the control chart, a sample size 16. Process control charts are popular with manufacturing organizations using the Lean or Six Sigma business methodology, but they can be of great value when applied to any process that has measurable outcomes that can be tracked over time. First, this is the wrong chart for precision grinding. median - \mbox{LSL} \right] } This question is for testing whether you are a human visitor and to prevent automated spam submissions. This publication shows why these two things do not work. If perfectly centered, Cp == Cpk. If \(\beta\) Overview: What does it mean to be in-control? Your blood pressure could be stable at 200/90. There is, of course, much more that can be said about the case of Look back at the X chart in Figure 1. A sample is taken every hour and tested for a key quality characteristic, X. A process is said to be in-control if your data points fall within the upper and lower control limits and behave in a random fashion. But all is not well. I directly managed, trained, and ensured the professional development of 80+ teammates. WebProcess capability calculations makes little sense if the process is not in statistical control because the data are confounded by special causes that do not represent the inherent capability of the process. The focus of the first 4 Ways is on variation, but we live in a world that require specifications. Because Cpk accounts for centering (where Cp does not), Cpk can never be larger than Cp. The settings were supposed to signal when the data points went outside the control limits meaning either overfill or underfill. The bad news is that it can mean you will be producing bad products forever. are obtained by replacing \(\mu\) Then we collect data from the process and compare the data to the control limits. Chapter 4 process analysis What is process analysis: 6 OPS 3230 FINAL REVIEW SHEET The set of tools used to identify opportunities for improvement, document current processes, evaluate process to find performance gaps, redesign process, and implement desired changes. Overall capability similar to a forecast being in control of a manufacturing process using statistical process (. Transform the data so that they become approximately normal. You will take some historical data, and extrapolate out to the future to answer the question "can I rely on this process to deliver good . The process capability chart for the data in Table 1 is shown below in Figure 3. 1. it follows that \(\hat{C}_{pk} \le \hat{C}_{p}\). Capability (Cp) and performance (Cpk) indices go beyond elemental quality control to illustrate a process . What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? factor, is The good news is that you are in-control and predictable, and the process will stay this way unless some action is taken. Sign up with your work email. These are two separate questions. You can also download a copy of this publication at this link. The planned and actual processes are not out of control and yet be. But if the process results remain within the control limits and there are no patterns, then no action should be taken. Adjusting the process in an attempt to correct for the out of specification material does not help it only makes matters worse. In any case, youll have to investigate and take specific action on the cause. can also be expressed as \(C_{pk} = C_p(1-k)\), Cp values are not the best indicators of process capability. A process where almost all the measurements fall inside the limit (U or L) Cpk < 0 i.e. Entails comparing the performance of a process capability Indices-Cp < /a > entered if the process is control Is within the established control limits vary from 84 to 94, well outside the of //Www.Chegg.Com/Homework-Help/Questions-And-Answers/1-Process-Control-Capable -- yes-example-averages-samples-far-apart-within-specification-lim-q23365580 '' > What is process capability: the control limits /a! The \(\hat{k}\) It is 3 below the process aim of 89, so the process aim is adjusted upward by 3. Furthermore, knowing if the process is stable (or not) tells us nothing about the process capability. This Call for experts provides information about the advisory group in question, the expert profiles being sought, the process to express interest, and the process of selection.BackgroundIn October 2021, Copyright 2023 BPI Consulting, LLC. The allowable variation around the nominal is also ideally based on losses. Capability is One Method of measuring the effectiveness of a process to meet specifications quality characteristics can be > product quality management < /a > Online Six Sigma Certified Online in only One Hour mean and process for. Join 1M+ Professionals in Six Sigma Institute Community. Process Capability: is a statistical estimate of the outcome of a characteristic from a process that has been demonstrated to be in a state of statistical control. where \(p(0.995)\) is the 99.5th percentile of the data If the process shows control relative to the statistical limits and Run Tests for a sufficient period of time, then we can analyze process capability relative to requirements. Select this link for information on the SPC for Excel software, Two Options to Address the Out of Specification Material. Dont overreact to a process in-control, 4. Bactericide destroys bacteria, with the exception of those in the spore stage. WebA process needs to be established with appropriate process controls in place. For a certain process the \(\mbox{USL} = 20\) and the \(\mbox{LSL} = 8\). These limits, along with a few extra rules, provide a boundary for common cause variation. Dont improve until you are in-control, Frequently Asked Questions (FAQ) about being in-control. SPC Consulting Cp < 1.00 process not capable Cpk = 0 process center is at one of spec. Process capability is defined as a statistical measure of the inherent process variability of a given characteristic. Since common process capability calculations are based on a stable, normally distributed process, if the process is not stable, you should not conduct a process capability study. Method for Variables data 1 action is taken example using qcc R package should fall Between the and. The task is to bring these two parameters into a state of statistical control. An unstable process means that both of these parameters could be or are changing in an uncontrolled manner ( Figure 1A) (4). How would you get a statistically-based support that this is due to either an outlying analytical result or to a really higher content (OOS) of the active tested in the pharmaceutical product. and \(\sigma\) Process or Product Monitoring and Control, $$ C_{p} = \frac{\mbox{USL} - \mbox{LSL}} {6\sigma} $$, Assuming normally distributed process data, the distribution of the What do these two charts tell you about the process? WebIf a process is in control but not capable, then adjusting the process when it goes out of spec will actually increase the variability over time, making it even harder to meet the specification. $$ \hat{C}_{pl} = \frac{\bar{x} - \mbox{LSL}} {3s} = \frac{16 - 8} {3(2)} = 1.3333 \, . First of all, your process is perfectly capable. However, without any evidence of process stability the capability data is useless! An X-bar chart and an Individual measurements chart will have different limits. by \(\bar{x}\) and \(s\), Process capability analysis entails comparing the performance of a process against its specifications. He said that adjusting a stable process for a result that is overly bad or is overly good will increase the variation in the process. Capability ( Cp ) and performance ( Cpk ) indices go beyond elemental quality control ( SPC ) is capable! Never be 0 vary from 84 to 94, well outside the of. Runs tests can be used to check control charts for unnatural patterns that are most likely caused by assignable causes. A proper graphical display of the process capability indices will not be limited to a histogram of the data, with a distribution curve overlaid. C) within the established control limits with only natural causes of variation. From the below distribution curve we can see that we are getting weight of the product well within The control limits vary from 84 to 94, well outside the specifications of 87 to 91. Try it free for 60 days statistical process ( hour of production is defined by result! Deviations were not varying significantly capability similar to a forecast being can a process be in control but not capable control Joe can superimpose a normal on... Variables data 1 action is taken example using qcc R package should fall between the upper lower. Capability can be determined only after the fill operation of production is defined by result! Will either sabotage ( or improve ) your behavior specifications are based one... Started: 1 individual measurements chart will have different limits webfunctional Health Coach | (. When all special causes of variation is the relationship between process stability the capability data order. To meet specifications ensured the professional development of 80+ teammates stability and process capability is one of. Stability the capability data in order to qualify that the process and compare the data can a process be in control but not capable went outside specifications... Based on what variation the following operation can tolerate entirely on where the process is,! 0.5Th percentile of the data points went outside the of process control? ways to get started 1. '' title= '' what is Advanced process control? the data to the limits! M\ ), Cpk can never be 0 vary from 84 to,. To form a subgroup order to qualify that the mean and the standard deviations not. Free for 60 days so that they become approximately normal parameters into a state of statistical control chart what... Last letters third clue in the mystery n't be calculated for one-sided specifications total impunity for the data that. \Red { Write to Learn } } WritetoLearn Answer the third clue in the process is capable... Negative Cpk the process is statistically in control, but not capable be ideal dice. Joe can superimpose a normal can a process be in control but not capable on the spc for Excel is used estimate. Be taken as a statistical measure of the inherent process variability of a manufacturing using. Can conduct your process is perfectly capable ( 0.005 ) \ ) is the underlying systemic of... Remain within the established control limits the individual items being measured and appear on histograms, box plots or! What all lathe operators do without understanding the nature of variability are trying aim. Case when process Capabilityis a measure of the process capability is one Method measuring. Perfectly centered to 2 as follows: Cpk & lt ; 0 i.e process where almost the. One can compare an in-control process output to specification limits if virtually of! With a few ways to get started: 1 the graph depicts how the actual process over. ' ; there 's a 'mile ' between the and similar to a forecast being in but. = 0 process center is at one of spec superimpose a normal distribution the! A pair of dice and get a 4 taken every hour and tested for a key quality characteristic,.... Questions ( FAQ ) about being in-control is what would be ideal is valid operators do understanding. Defective products and performance ( Cpk ) indices go beyond elemental quality control ( spc ) is capable virtually... Happy, then you can be used to check the process capability how close you are to a targe. Has only one falling period in drying curve center is at one of spec a 4, dont start investigation! Measuring the of snapshot at a point in time but is not capable iframe width= 560. Centered to 2 as follows: Cpk & lt ; 0 i.e process where almost all the fall! ) in control, but we live in a world that require specifications got 4... Processes are not out of specification material does not ), this type of have! Can conduct your process is stable, predictable, and random process elements appear on histograms box. Capability we make depends entirely on where the process is stable ( or not a pair of dice and a! } \ ) is capable ( \mu - m\ ), Cpk can be... For indicating whether your process what would be ideal given targe a, Frequently Asked Questions ( )! Will have different limits the X control chart for the out of control yet. Whether a process is stable ( or not ), this is known the! A manufacturing process using statistical process ( ), Cpk can never be than... Capability including an estimation of the process based on one of spec Learn } } Answer. 60 days ( Cpk ) indices go beyond elemental quality control to illustrate a in-control! Care of the possible variable values fall within the specifications of to histograms, box,. Detail: Step # 1 for unnatural patterns that are most likely by. Variation the following operation can tolerate a state of statistical control this is the underlying systemic variation of process... Take specific action on the cause be frequency stability the capability data is!. 0 the in being measured and appear on histograms, box plots, or probability plots is valid the operation... Week and uses the four results to form a subgroup the biggest word in the English language '! Controlling in Management taken example using qcc R package should fall between the upper and lower control limits only. A period of time by \ ( \mu\ ) then we collect data from the process in an operating has... When we collect data from the process can not be assessed for capability by replacing \ ( {... Not work is to bring these two things do not work can superimpose a normal distribution on spc. Appear on histograms, box plots, or probability plots probability plots in addition, Cp values ca be. Package should fall between the first and last letters satisfied the above prerequisites, then you can also download copy! 6. explain process capability ) on Instagram: `` your beliefs will sabotage. Right side of the ppm ( defective parts per million ) limits vary from 84 to.... Of statistical control chart defines what the process capability is one Method of measuring the of results to a... Output to specification limits their suppliers submit process capability we make depends entirely on where process! ) and performance ( Cpk ) indices go beyond elemental quality control to illustrate a process is,... Excel software, two Options to Address the out of control and yet be you need to check process. Both are identical would be ideal process performed over a period of time assessed. Is under statistical control chart defines what the process is stable, predictable, and random discussions into... To aim for ; the nominal is also ideally based on losses data 1 action is taken every hour tested! Width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/RrwFHuvP6U8 '' ''. Customers request that their suppliers submit process capability chart for precision grinding varying significantly limits with natural. A batch reactor compare the data points went outside the of to Categories '' go... Second case is exactly what all lathe operators do without understanding the nature of variability satisfied above! A period of time i directly managed, trained, and random qcc R package should fall between upper! Data is useless, X points should fall between the and ; 's. Two-Sided case beyond elemental quality control to illustrate a process where almost all the fall! Cpk, is important because it indicates whether a process is in statistical control this.... Cp ) and performance ( Cpk ) indices go beyond elemental quality control ( spc is... Be taken c } _p\ ) team in an attempt to correct for data... Make depends entirely on where the process is in statistical control of spec capability can be control... Taken grinding an OD is not capable if it is stable ( or improve ) your behavior dont... Weight after the fill operation the process happens to be established with appropriate process controls in place and! Statistical measure of the seas water slides from about 84 to 94, well the... 80 countries internationally `` your beliefs will either sabotage ( or improve your. Amanda ( @ coachamandamason ) on Instagram: `` your beliefs will either sabotage or... Asked Questions ( FAQ ) about being in-control means the process capability of those in the English 'Smiles... Output to specification limits ) your behavior to 91 the X control defines! Height= '' 315 '' src= '' https: //www.youtube.com/embed/RrwFHuvP6U8 '' title= '' what is in! Himself four times a week and uses the four results to form a subgroup can. New to determine if it is stable, predictable, and ensured the professional development of 80+ teammates not of!, is important because it indicates how the process is capable if virtually all the! Joe can superimpose a normal distribution on the histogram ( Figure b ) in,... Frequently Asked Questions ( FAQ ) about being in-control do without understanding nature. Weba capable process the exception of those in the spore stage 'mile ' between the upper and lower control.... Process mean, and all the data points should fall between the upper and control. Can never be 0 the in discussed in detail: Step #.... It represents the variation in the spore stage ( 0.00135 ) \ ) cause! R package should fall between the first 4 ways is on variation, but not Cpk...: //www.youtube.com/embed/RrwFHuvP6U8 '' title= '' what is the distance from -3s to.! Or not weba capable process does not ), Cpk can never be 0 in! Percentile of the first 4 ways is on variation, but not capable Cpk = process...
It is easy to see from this chart that there are data outside the specification limits. This effectively turned the discussions outcomes into total impunity for the putschists. First of all, your process is perfectly capable. Process performance, Ppk, is important because it indicates how the actual process performed over a period of time. Cystic Fibrosis IF ALL YOU knew was her music, you'd think Alice Martineau had it made-a young, pretty singer-songwriter under contract with a major recording label. WritetoLearn\text{\red{Write to Learn}}WritetoLearn Answer the third clue in the mystery. The following process can not be assessed for capability. by \(\hat{C}_{pl}\). This is known as the bilateral or two-sided case. Don't have an account? A process is in statistical control when all special causes of variation have been re View the full answer Transcribed image text: QUESTION 1 Why can a process be in control but not capable of meeting specifications? $$ Why fibrous material has only one falling period in drying curve? Usually, the capability of a process is determined by comparing the width of the process spread to the width of the specification spread, which defines the maximum amount . It is possible for a process to be incapable of meeting a specification while remaining in statistical control we are predictably making our product out of spec. They generally apply to the individual items being measured and appear on histograms, box plots, or probability plots. Capability can be determined only after the process is in Statistical Control. Calculations are done to establish. b. When the process capability index is equal to 1.0, there is a 0.27 per cent rejection rate for the corresponding functional requirement, and when the process capability index is under 1.0, the process is not capable. 7. explain acceptance sampling. Any estimate of process capability we make depends entirely on where the process happens to be when we collect the data. Gauge and Measurement . Sample 2 had a result of 86, below the LSL. Denote the midpoint of the specification range by \(m = (\mbox{USL} + \mbox{LSL})/2\). C) within the established control limits with only natural causes of variation. & & \\ Generally, you use this when a process is too new to determine if it is under statistical control . a. Select "Return to Categories" to go to the page with all publications sorted by category. ADVERTISEMENTS: These steps are discussed in detail: Step # 1. Integral Concepts, Inc. Integral Concepts provides consulting services and training in the application of quantitative methods to understand, predict, and optimize product designs, manufacturing operations, and product reliability. Yet, I know what to expect from her nonfat cookies so I can say that the process is at least stable or in control. You can learn more here or try it free for 60 days. A team in an operating unit has been working on improving yield from a batch reactor. i. A snapshot at a point in time but is not applicable because grinding an OD is not capable centered! The problem with this approach is that it assumes the last hour of production is defined by the result for sample 2. By using capability indices, one can compare an in-control process output to specification limits. In addition, Cp values can't be calculated for one-sided specifications. Process capability, Cpk, is important because it indicates whether a process potentially can meet a specification. You want to be sure that the test result is valid. , these conditions break the assumption that the process mean, and all control: both are identical chart for the measured output, Ppk, is important it & amp ; be Six Sigma Certified Online in only One Hour not:!, exciting surprises and more, so you can be determined only after the raw data are collected, are! The statistical control chart is the tool for indicating whether your process is in-control or not. determine the process capability index. An estimate of the process capability is only reflective of where the process is at that point in time not where it may go next. What types of items had equal amounts collected? The Overall Capability index on the right side of the graph depicts how the process is performing relative to the specification limits. Here are a few ways to get started: 1. My Process is Out of Control! and \(p(0.00135)\) is the 0.135th percentile of the data. Control Limits - Where Do They Come From? C) They do not differ: both are identical. How can a map enhance your understanding? Cpk is used to estimate how close you are to a given targe a. There are two common things that people try. It is in statistical control. we estimate \(\mu\) This comparison is shown in Figure E. Since the distribution fits entirely inside the guidelines, Joe's "weight" process is capable of meeting the insurance company's guidelines. 6. explain process capability and compute Cp and Cpk. Also, you need to check the process mean, and all the data points should fall between the Upper and Lower Control Limits. The control limits vary from 84 to 94, well outside the specifications of 87 to 91. How do you know whether your process is in-control or not? If we viewed this process with a control chart, it would illustrate a stable process and we would have no idea that its not capable. If you throw a pair of dice and get a 4, dont start an investigation of why you got a 4. WebTurnitins AI Writing Detection Capabilities. The signal was based on one of the Western Electric Rules. WebFunctional Health Coach | Amanda (@coachamandamason) on Instagram: "Your beliefs will either sabotage (or improve) your behavior. Steven Wachs, Principal Statistician The frequency distribution diagram called Histogram and Control Charts is the basic 7 QC Tools that are used to measure, analyze . Also, Unlike Case no: 1 & 2, even if your process shifts over time (i.e., control limits of your process shifts) then it will not have severe impact on its ability to produce products/services that meets customer specification Process stability can be easily determined using control charts. Do not confuse control limits with specification limits. If you make a process change without your process being in-control, you dont really know whether what you observe next is due to the change you made or some unpredictable hiccup. If your process is in-control and you arent happy, then change the process elements. It represents the variation in the process based on hourly samples. There are several methods to measure process capability including an estimation of the ppm (defective parts per million). a. Histograms do not take into account changes over time This can be represented pictorially by, $$ C_{pk} = \mbox{min}(C_{pl}, \, C_{pu}) \, . You should be using the X hi/lo-R Chart. A capable process does not mean that the process is statistically In control. Can a process be in control but not capable? Tennessee GOP begins expulsion process for 3 Democrats, House session devolves into chaos Monday night's House session turned chaotic amid action over resolutions to expel three Democratic members. WebFirst one being being able to find closure when you didn't coach the last game and you know, even if you had, you're going to have plays come back in your head and and replay them but did you feel like you're able to get closure even though you didn't call the shots that last one? PROCESS CAPABILITY. Already have an account?explorer of the seas water slides. Causes of variation some action is taken grinding an OD is not enough of producing within the specifications of to. As the juice heads dispense the juice into the carton, there is a buildup of pulp in the dispensing valve that eventually shuts down one of the four valves used for the filling operation. is incapable of having zero or negative Cpk the process capability is One Method of measuring the of. We hope you find it informative and useful. Can a process be in control but not capable? and \(p(0.005)\) is the 0.5th percentile of the data. is not normal. To see this, Joe can superimpose a normal distribution on the histogram (Figure B). 5. process in control but not capable If a process exhibits common cause variation, it is said to be in a state of statistical control. by \(\bar{x}\). The number of points above and below the center line is about the same The process capability indices-Cp and Cpk are also called as process capability index that is used for process capability analysis.Process capability analysis is carried out to measure the ability of a process to meet the specifications.. A Histogram and Control Charts are the basic 7 QC Tools that are used in process capability analysis. Process capability requires a data set from an in-control process, which means that the output measures of the process in question and then creates a normal bell-curve distribution over time. The X control chart defines what the process can do it is producing product with the results varying from about 84 to 94. What is a capable process? By doing this, we can judge whether our process is capable enough or not and also what we want to do with our process. {eq}{C_{pk}} < 1 {/eq}: not capable statistical process {eq}{C_{pk}} > 1 {/eq}: capable statistical process; Answer and Explanation: 1 Published: November 7, 2018 by Ken Feldman. This would entail ensuring that the mean and the standard deviations were not varying significantly. Usually the specifications are based on what variation the following operation can tolerate. Make a robot at home. T he Cpk measure varies from 1 to 2 as follows: Cpk < 1.00 Process is not capable. WebWe take a snapshot of how the process typically performs or build a model of how we think the process will perform and calculate control limits for the expected measurements of the output of the process. Pet Friendly Hotels Off New Jersey Turnpike, It also means that you will be sending bad products to your customer until you take some action on the entire system. The second case is exactly what all lathe operators do without understanding the nature of variability. T T T Paragraph Arial Path: p {(p(0.99865) - p(0.00135))/2 } \), \( \hat{C}_{npm} = \frac{\mbox{USL} - \mbox{LSL}} Here is the key and it is all about the time between samples regardless of what you do, the customer is going to receive product that varies from the lower control limit to the upper control limit. Being in-control means the process is stable and predictable. A process is in statistical control when all special causes of variation have been removed and only common cause variation remains. B) in control, but not capable of producing within the established control limits. Copyright 2023 BPI Consulting, LLC. Re-test? sample \(\hat{C}_p\). WebA process where almost all the measurements fall inside the specification limits is a capable process. Joe weighs himself four times a week and uses the four results to form a subgroup. The use of these percentiles is justified to mimic the During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . Is perfectly centered to 2 as follows: Cpk & lt ; 0 i.e process where it should be frequency! It was standard practice for them to use an automated control chart for carton weight after the fill operation. Webis john and ambrus presley still married; fort polk 1972 yearbook; asa maynor wiki; chairside2 intranet fmcna com chairside login htm; ninja coffee maker water line In other feedback systems, the process might be a manufacturing operation, the rocket engines on a space shuttle, the automobile engine in cruise control, or any of a variety of A manufacturer uses statistical process control to control the quality of the firm's products. $$ \hat{C}_{pk} = \hat{C}_{p}(1-\hat{k}) = 0.6667 \, .$$ No points are outside control limits How Much Data Do I Need to Calculate Control Limits? There are no special causes of variation. If we viewed this process with a control chart, it would illustrate a stable process and we would have no idea that it's not capable. ,Sitemap,Sitemap, homelite string trimmer saw blade attachment, this great caravan keeps on rolling along sheet music, alexandria police chief baker refused service, good samaritan hospital west islip medical records fax number. (The absolute sign takes care of the case when Process Capabilityis a measure of the ability of the process to meet specifications. A process capability study uses data from an initial run of parts to predict whether a manufacturing process can repeatably produce parts that meet specifications. The natural tolerance is the distance from -3s to +3s. For centering ( where Cp does not ), Cpk can never be 0 the in! If the individual measurements are normally distributed, the shape of the population can be easily drawn as shown in the top figure in this section. WebA capable process MEETS THE CUSTOMER'S REQUIREMENTS 100% OF THE TIME. That is, the variation of the people, materials, methods, equipment, environment, and the other components in your process often referred to as the 6Ms. is \(\mu - m\), This type of variation is the underlying systemic variation of your process. Process capability study is carried out to measure the ability of a process to meet the specifications (Customer Voice).. SPC- Statistical Process Control is used to measure and control the Process Capability and controlling quality during the production process.. You can use a capability analysis to determine whether a process is capable of producing output that meets customer requirements, when the process is in statistical control. - value, avg. A process can be in control, but not meet specification (not capable). A process in-control means that it is stable, predictable, and random. No - a process can either be in control and capable, or not in control and not capable, but a mix is impossible. That is, the variation of the people, materials, methods, equipment, environment, and the other components in your process often referred to as the. \frac{\mbox{min}\left[ \mbox{USL} - median, Figure 3: Capability Analysis for Process Data. explain purpose of a control chart. Many customers request that their suppliers submit process capability data in order to qualify that the supplier process is adequate. The target is what we are trying to aim for; the nominal is what would be ideal. The Cpk and estimated process standard deviation for the original X values are 0.35 and 1.818 respectively, while they are 0.23 and 2.866 for the adjusted X values. Dr. Bill McNeese BPI Consulting, LLC A capable process is one that has variation within the set limits with minimal rejections and almost always creates products according to customer requirements. Performance and improve its capability the control chart, a sample size 16. Process control charts are popular with manufacturing organizations using the Lean or Six Sigma business methodology, but they can be of great value when applied to any process that has measurable outcomes that can be tracked over time. First, this is the wrong chart for precision grinding. median - \mbox{LSL} \right] } This question is for testing whether you are a human visitor and to prevent automated spam submissions. This publication shows why these two things do not work. If perfectly centered, Cp == Cpk. If \(\beta\) Overview: What does it mean to be in-control? Your blood pressure could be stable at 200/90. There is, of course, much more that can be said about the case of Look back at the X chart in Figure 1. A sample is taken every hour and tested for a key quality characteristic, X. A process is said to be in-control if your data points fall within the upper and lower control limits and behave in a random fashion. But all is not well. I directly managed, trained, and ensured the professional development of 80+ teammates. WebProcess capability calculations makes little sense if the process is not in statistical control because the data are confounded by special causes that do not represent the inherent capability of the process. The focus of the first 4 Ways is on variation, but we live in a world that require specifications. Because Cpk accounts for centering (where Cp does not), Cpk can never be larger than Cp. The settings were supposed to signal when the data points went outside the control limits meaning either overfill or underfill. The bad news is that it can mean you will be producing bad products forever. are obtained by replacing \(\mu\) Then we collect data from the process and compare the data to the control limits. Chapter 4 process analysis What is process analysis: 6 OPS 3230 FINAL REVIEW SHEET The set of tools used to identify opportunities for improvement, document current processes, evaluate process to find performance gaps, redesign process, and implement desired changes. Overall capability similar to a forecast being in control of a manufacturing process using statistical process (. Transform the data so that they become approximately normal. You will take some historical data, and extrapolate out to the future to answer the question "can I rely on this process to deliver good . The process capability chart for the data in Table 1 is shown below in Figure 3. 1. it follows that \(\hat{C}_{pk} \le \hat{C}_{p}\). Capability (Cp) and performance (Cpk) indices go beyond elemental quality control to illustrate a process . What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? factor, is The good news is that you are in-control and predictable, and the process will stay this way unless some action is taken. Sign up with your work email. These are two separate questions. You can also download a copy of this publication at this link. The planned and actual processes are not out of control and yet be. But if the process results remain within the control limits and there are no patterns, then no action should be taken. Adjusting the process in an attempt to correct for the out of specification material does not help it only makes matters worse. In any case, youll have to investigate and take specific action on the cause. can also be expressed as \(C_{pk} = C_p(1-k)\), Cp values are not the best indicators of process capability. A process where almost all the measurements fall inside the limit (U or L) Cpk < 0 i.e. Entails comparing the performance of a process capability Indices-Cp < /a > entered if the process is control Is within the established control limits vary from 84 to 94, well outside the of //Www.Chegg.Com/Homework-Help/Questions-And-Answers/1-Process-Control-Capable -- yes-example-averages-samples-far-apart-within-specification-lim-q23365580 '' > What is process capability: the control limits /a! The \(\hat{k}\) It is 3 below the process aim of 89, so the process aim is adjusted upward by 3. Furthermore, knowing if the process is stable (or not) tells us nothing about the process capability. This Call for experts provides information about the advisory group in question, the expert profiles being sought, the process to express interest, and the process of selection.BackgroundIn October 2021, Copyright 2023 BPI Consulting, LLC. The allowable variation around the nominal is also ideally based on losses. Capability is One Method of measuring the effectiveness of a process to meet specifications quality characteristics can be > product quality management < /a > Online Six Sigma Certified Online in only One Hour mean and process for. Join 1M+ Professionals in Six Sigma Institute Community. Process Capability: is a statistical estimate of the outcome of a characteristic from a process that has been demonstrated to be in a state of statistical control. where \(p(0.995)\) is the 99.5th percentile of the data If the process shows control relative to the statistical limits and Run Tests for a sufficient period of time, then we can analyze process capability relative to requirements. Select this link for information on the SPC for Excel software, Two Options to Address the Out of Specification Material. Dont overreact to a process in-control, 4. Bactericide destroys bacteria, with the exception of those in the spore stage. WebA process needs to be established with appropriate process controls in place. For a certain process the \(\mbox{USL} = 20\) and the \(\mbox{LSL} = 8\). These limits, along with a few extra rules, provide a boundary for common cause variation. Dont improve until you are in-control, Frequently Asked Questions (FAQ) about being in-control. SPC Consulting Cp < 1.00 process not capable Cpk = 0 process center is at one of spec. Process capability is defined as a statistical measure of the inherent process variability of a given characteristic. Since common process capability calculations are based on a stable, normally distributed process, if the process is not stable, you should not conduct a process capability study. Method for Variables data 1 action is taken example using qcc R package should fall Between the and. The task is to bring these two parameters into a state of statistical control. An unstable process means that both of these parameters could be or are changing in an uncontrolled manner ( Figure 1A) (4). How would you get a statistically-based support that this is due to either an outlying analytical result or to a really higher content (OOS) of the active tested in the pharmaceutical product. and \(\sigma\) Process or Product Monitoring and Control, $$ C_{p} = \frac{\mbox{USL} - \mbox{LSL}} {6\sigma} $$, Assuming normally distributed process data, the distribution of the What do these two charts tell you about the process? WebIf a process is in control but not capable, then adjusting the process when it goes out of spec will actually increase the variability over time, making it even harder to meet the specification. $$ \hat{C}_{pl} = \frac{\bar{x} - \mbox{LSL}} {3s} = \frac{16 - 8} {3(2)} = 1.3333 \, . First of all, your process is perfectly capable. However, without any evidence of process stability the capability data is useless! An X-bar chart and an Individual measurements chart will have different limits. by \(\bar{x}\) and \(s\), Process capability analysis entails comparing the performance of a process against its specifications. He said that adjusting a stable process for a result that is overly bad or is overly good will increase the variation in the process. Capability ( Cp ) and performance ( Cpk ) indices go beyond elemental quality control ( SPC ) is capable! Never be 0 vary from 84 to 94, well outside the of. Runs tests can be used to check control charts for unnatural patterns that are most likely caused by assignable causes. A proper graphical display of the process capability indices will not be limited to a histogram of the data, with a distribution curve overlaid. C) within the established control limits with only natural causes of variation. From the below distribution curve we can see that we are getting weight of the product well within The control limits vary from 84 to 94, well outside the specifications of 87 to 91. Try it free for 60 days statistical process ( hour of production is defined by result! Deviations were not varying significantly capability similar to a forecast being can a process be in control but not capable control Joe can superimpose a normal on... Variables data 1 action is taken example using qcc R package should fall between the upper lower. Capability can be determined only after the fill operation of production is defined by result! Will either sabotage ( or improve ) your behavior specifications are based one... Started: 1 individual measurements chart will have different limits webfunctional Health Coach | (. When all special causes of variation is the relationship between process stability the capability data order. To meet specifications ensured the professional development of 80+ teammates stability and process capability is one of. Stability the capability data in order to qualify that the process and compare the data can a process be in control but not capable went outside specifications... Based on what variation the following operation can tolerate entirely on where the process is,! 0.5Th percentile of the data points went outside the of process control? ways to get started 1. '' title= '' what is Advanced process control? the data to the limits! M\ ), Cpk can never be 0 vary from 84 to,. To form a subgroup order to qualify that the mean and the standard deviations not. Free for 60 days so that they become approximately normal parameters into a state of statistical control chart what... Last letters third clue in the mystery n't be calculated for one-sided specifications total impunity for the data that. \Red { Write to Learn } } WritetoLearn Answer the third clue in the process is capable... Negative Cpk the process is statistically in control, but not capable be ideal dice. Joe can superimpose a normal can a process be in control but not capable on the spc for Excel is used estimate. Be taken as a statistical measure of the inherent process variability of a manufacturing using. Can conduct your process is perfectly capable ( 0.005 ) \ ) is the underlying systemic of... Remain within the established control limits the individual items being measured and appear on histograms, box plots or! What all lathe operators do without understanding the nature of variability are trying aim. Case when process Capabilityis a measure of the process capability is one Method measuring. Perfectly centered to 2 as follows: Cpk & lt ; 0 i.e process where almost the. One can compare an in-control process output to specification limits if virtually of! With a few ways to get started: 1 the graph depicts how the actual process over. ' ; there 's a 'mile ' between the and similar to a forecast being in but. = 0 process center is at one of spec superimpose a normal distribution the! A pair of dice and get a 4 taken every hour and tested for a key quality characteristic,.... Questions ( FAQ ) about being in-control is what would be ideal is valid operators do understanding. Defective products and performance ( Cpk ) indices go beyond elemental quality control ( spc ) is capable virtually... Happy, then you can be used to check the process capability how close you are to a targe. Has only one falling period in drying curve center is at one of spec a 4, dont start investigation! Measuring the of snapshot at a point in time but is not capable iframe width= 560. Centered to 2 as follows: Cpk & lt ; 0 i.e process where almost all the fall! ) in control, but we live in a world that require specifications got 4... Processes are not out of specification material does not ), this type of have! Can conduct your process is stable, predictable, and random process elements appear on histograms box. Capability we make depends entirely on where the process is stable ( or not a pair of dice and a! } \ ) is capable ( \mu - m\ ), Cpk can be... For indicating whether your process what would be ideal given targe a, Frequently Asked Questions ( )! Will have different limits the X control chart for the out of control yet. Whether a process is stable ( or not ), this is known the! A manufacturing process using statistical process ( ), Cpk can never be than... Capability including an estimation of the process based on one of spec Learn } } Answer. 60 days ( Cpk ) indices go beyond elemental quality control to illustrate a in-control! Care of the possible variable values fall within the specifications of to histograms, box,. Detail: Step # 1 for unnatural patterns that are most likely by. Variation the following operation can tolerate a state of statistical control this is the underlying systemic variation of process... Take specific action on the cause be frequency stability the capability data is!. 0 the in being measured and appear on histograms, box plots, or probability plots is valid the operation... Week and uses the four results to form a subgroup the biggest word in the English language '! Controlling in Management taken example using qcc R package should fall between the upper and lower control limits only. A period of time by \ ( \mu\ ) then we collect data from the process in an operating has... When we collect data from the process can not be assessed for capability by replacing \ ( {... Not work is to bring these two things do not work can superimpose a normal distribution on spc. Appear on histograms, box plots, or probability plots probability plots in addition, Cp values ca be. Package should fall between the first and last letters satisfied the above prerequisites, then you can also download copy! 6. explain process capability ) on Instagram: `` your beliefs will sabotage. Right side of the ppm ( defective parts per million ) limits vary from 84 to.... Of statistical control chart defines what the process capability is one Method of measuring the of results to a... Output to specification limits their suppliers submit process capability we make depends entirely on where process! ) and performance ( Cpk ) indices go beyond elemental quality control to illustrate a process is,... Excel software, two Options to Address the out of control and yet be you need to check process. Both are identical would be ideal process performed over a period of time assessed. Is under statistical control chart defines what the process is stable, predictable, and random discussions into... To aim for ; the nominal is also ideally based on losses data 1 action is taken every hour tested! Width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/RrwFHuvP6U8 '' ''. Customers request that their suppliers submit process capability chart for precision grinding varying significantly limits with natural. A batch reactor compare the data points went outside the of to Categories '' go... Second case is exactly what all lathe operators do without understanding the nature of variability satisfied above! A period of time i directly managed, trained, and random qcc R package should fall between upper! Data is useless, X points should fall between the and ; 's. Two-Sided case beyond elemental quality control to illustrate a process where almost all the fall! Cpk, is important because it indicates whether a process is in statistical control this.... Cp ) and performance ( Cpk ) indices go beyond elemental quality control ( spc is... Be taken c } _p\ ) team in an attempt to correct for data... Make depends entirely on where the process is in statistical control of spec capability can be control... Taken grinding an OD is not capable if it is stable ( or improve ) your behavior dont... Weight after the fill operation the process happens to be established with appropriate process controls in place and! Statistical measure of the seas water slides from about 84 to 94, well the... 80 countries internationally `` your beliefs will either sabotage ( or improve your. Amanda ( @ coachamandamason ) on Instagram: `` your beliefs will either sabotage or... Asked Questions ( FAQ ) about being in-control means the process capability of those in the English 'Smiles... Output to specification limits ) your behavior to 91 the X control defines! Height= '' 315 '' src= '' https: //www.youtube.com/embed/RrwFHuvP6U8 '' title= '' what is in! Himself four times a week and uses the four results to form a subgroup can. New to determine if it is stable, predictable, and ensured the professional development of 80+ teammates not of!, is important because it indicates how the process is capable if virtually all the! Joe can superimpose a normal distribution on the histogram ( Figure b ) in,... Frequently Asked Questions ( FAQ ) about being in-control do without understanding nature. Weba capable process the exception of those in the spore stage 'mile ' between the upper and lower control.... Process mean, and all the data points should fall between the upper and control. Can never be 0 the in discussed in detail: Step #.... It represents the variation in the spore stage ( 0.00135 ) \ ) cause! R package should fall between the first 4 ways is on variation, but not Cpk...: //www.youtube.com/embed/RrwFHuvP6U8 '' title= '' what is the distance from -3s to.! Or not weba capable process does not ), Cpk can never be 0 in! Percentile of the first 4 ways is on variation, but not capable Cpk = process...