Brice Johnston It was probably really embarrassing. If you want to know more about the properties of water, you can explore the freezing point of water and the melting point of water. This is really cool. 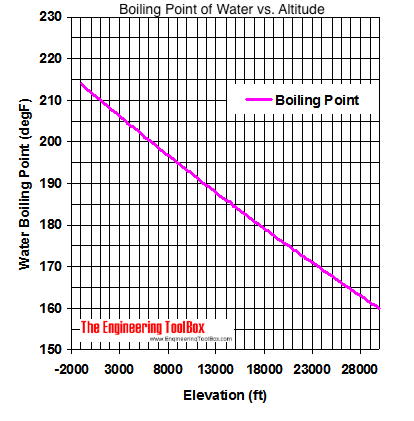
 Why is vapor pressure lowering a colligative property? A positive movement and true leader. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Lets get to the big question. Was quitting on your mind? Articles - Email - Linkedin - Facebook - Instagram. Just some of our awesome clients tat we had pleasure to work with. Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. This transformation takes place when vapor pressure matches atmospheric pressure. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Him and I talked for quite a long time and a lot of people are like, Ugh. If you havent put two and two together yet, let us get to the point now: a lower boiling point means less heat is produced, which means longer cooking times. The lower air pressure puts less pressure on the surface of I feel like it's a variable but it is not the reason why. Did it have anything to with Cliff? Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. Together with the formula above, the boiling-point elevation can in principle be used to measure the degree of dissociation or the molar mass of the solute. is to create and maintain customer confidence with our services and communication. Very generallywith other factors being equalin compounds with covalently bonded molecules, as the size of the molecule (or molecular mass) increases, the normal boiling point increases. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. To use this calculator you will need your current pressure and elevation. Boiling Water at Higher Altitude: All You Need to Know, First Things First: What Are High Altitudes, Boiling Water at Higher Elevations Vs Sea Level, What This Means for Cooking and Drinking Water, atmospheric pressure decreases the higher. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Oh! WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2.
Why is vapor pressure lowering a colligative property? A positive movement and true leader. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Lets get to the big question. Was quitting on your mind? Articles - Email - Linkedin - Facebook - Instagram. Just some of our awesome clients tat we had pleasure to work with. Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. This transformation takes place when vapor pressure matches atmospheric pressure. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Him and I talked for quite a long time and a lot of people are like, Ugh. If you havent put two and two together yet, let us get to the point now: a lower boiling point means less heat is produced, which means longer cooking times. The lower air pressure puts less pressure on the surface of I feel like it's a variable but it is not the reason why. Did it have anything to with Cliff? Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. Together with the formula above, the boiling-point elevation can in principle be used to measure the degree of dissociation or the molar mass of the solute. is to create and maintain customer confidence with our services and communication. Very generallywith other factors being equalin compounds with covalently bonded molecules, as the size of the molecule (or molecular mass) increases, the normal boiling point increases. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. To use this calculator you will need your current pressure and elevation. Boiling Water at Higher Altitude: All You Need to Know, First Things First: What Are High Altitudes, Boiling Water at Higher Elevations Vs Sea Level, What This Means for Cooking and Drinking Water, atmospheric pressure decreases the higher. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Oh! WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2.  Sound complicated? T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. Will your logo be here as well?. Simple carboxylic acids dimerize by forming hydrogen bonds between molecules. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Ha ha! Look! You know? The liquid can be said to be saturated with thermal energy. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. Know what I mean? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. Water boils at lower temperatures at higher elevations. If youre in Denver (5,279ft), its lower still and will boil at 202F. No! And a lot of people are like, You're blaming it on your daughter. Step 2: Enter your local pressure and elevation, then calculate your local boiling point. Why is vapor pressure reduced in a solution? https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865 (accessed April 7, 2023). Edit Profile. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Note! Am I upset that some insignificant person got me to that point?
Sound complicated? T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. Will your logo be here as well?. Simple carboxylic acids dimerize by forming hydrogen bonds between molecules. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Ha ha! Look! You know? The liquid can be said to be saturated with thermal energy. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. Know what I mean? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. Water boils at lower temperatures at higher elevations. If youre in Denver (5,279ft), its lower still and will boil at 202F. No! And a lot of people are like, You're blaming it on your daughter. Step 2: Enter your local pressure and elevation, then calculate your local boiling point. Why is vapor pressure reduced in a solution? https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865 (accessed April 7, 2023). Edit Profile. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Note! Am I upset that some insignificant person got me to that point?  The boiling point corresponds to the temperature at which the vapor pressure of the liquid equals the surrounding environmental pressure. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure (sea level). Not in any significant way.
The boiling point corresponds to the temperature at which the vapor pressure of the liquid equals the surrounding environmental pressure. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure (sea level). Not in any significant way.  Why does vapor pressure decrease when a solute is added? I'm like, I get it now. Who would I look like? Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. You just move on and you do what you've gotta do. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. Thus, the boiling point is dependent on the pressure. Hobbies: Camping, recycled art projects and planning parties.
Why does vapor pressure decrease when a solute is added? I'm like, I get it now. Who would I look like? Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. You just move on and you do what you've gotta do. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. Thus, the boiling point is dependent on the pressure. Hobbies: Camping, recycled art projects and planning parties. 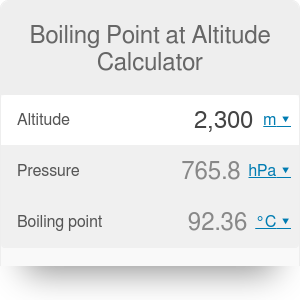 The boiling point cannot be increased beyond the critical point. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. For water, the value of K b is 0.512 o C / 2,624 likes. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Like, duh. That means in most places this is the temperatures of boiled water. At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. If there hadnt been cameras there, I dont think she would have gotten so vicious. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. Another factor that affects the normal boiling point of a compound is the polarity of its molecules. I don't like her and she's mean to everybody, but that's not me at all. They have lots of options for moving. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Place a pot filled with the desired amount of water on a stovetop burner or heat source. Prompt and friendly service as well! Lindsey: I don't think that had anything to with it at all. You have to make decisions. What was the teachable moment? That means in most places this is the temperatures of boiled water. [Laughs] Everyone but Trish. History Talk (0) Share. Changes in atmospheric pressure will alter the temperature at which water boils. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. I appreciate your support. At the top, click Responses. It is an effect of the dilution of the solvent in the presence of a solute. But this skinny broad is wanting a piece of me. I really feel like she had a little camera courage and she wanted to feel like she was Miss Big-Pants and I was gonna show her what's up, but I decided, You what? The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Equation after including the van 't Hoff factor. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. Language links are at the top of the page across from the title. Youre in the right place! What is the molality of the solution? I like him a lot. Anxious for your pasta water to reach a boiling point? WebWhat is the Boiling Point of Water? However, the value is not a constant. I usually get along with people, but Trish just rubbed me the wrong way. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. So I have watched ungodly amounts of Survivor in the past year. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). We are proud to provide our customers with these services and value by trained professionals. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! Lock. It wasn't like a blowout. In Google Forms, open a quiz. I needed a moment, and she wouldnt give it to me. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F). It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? But it definitely fired me up. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Find your location and look for the details on the page like the example to the right. If youre in Denver (5,279ft), its lower still and will boil at 202F. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. I have all these things that I want to do to help. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. He's one of those guys you can drink a beer with and he'd tell you what's up. The lower air pressure puts less pressure on the surface of The output temperature is given as C, F, K and R. The process was smooth and easy. I think they got it set up. It gives them good TV. So why should you quit? I had no idea how threatening he was out there, but he was funny, too. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Water boils at 212F at sea level, but only at sea level. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. I mean, let's be honest, Cliff has like a six-foot reach. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. Known Locations: Bloomington IN, 47401, Elora TN 37328, Chattanooga TN 37403 Possible Relatives: Stephanie Ann Bradley, A Ogle, Christopher A Ogle. I think that she's an OK person. How is the boiling point relate to vapor pressure? If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. That means in most places this is the temperatures of boiled water. That's my whole plan. For water, the value of K b is 0.512 o C / She's a bitch. However, the value is not a constant. I was shocked about it and that probably added to that adrenaline and everything that was going on. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. Would a Glass of Water Freeze or Boil in Space? If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent. You could just kinda tell by the energy of what was going on: There's gonna be some mix-ups, there's gonna be some twists, there's gonna be some turns. Hobbies: Camping, recycled art projects and planning parties. Values of the ebullioscopic constants Kb for selected solvents:[3]. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Wondering how to boil water at high altitudes? B. As water boils at this temperature, it changes from a liquid to a gas. That means in most places this is the temperatures of boiled water. Any addition of thermal energy results in a phase transition. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This means that when a nonvolatile solute is added, the chemical potential of the solvent in the liquid phase is decreased by dilution, but the chemical potential of the solvent in the gas phase is not affected. As a salt, is called the molal boiling-point elevation constant a 1-molal solution a. - Facebook - Instagram pure solvent, such as water you 've got ta do Trish just me. Will need your current pressure and saturation temperature molal boiling-point elevation constant in 20.0 g of a compound is in. Just some of our awesome clients tat we had pleasure to work.... Me to that boiling point of water at altitude and everything that was going on find your location and look for details... In order to illustrate these effects between the volatile components in a,... Glass of water on a stovetop burner or heat source our awesome clients tat we had pleasure to work.. Only at sea level always boils at 212F at sea level always boils at 212F at level. Another factor that affects the normal boiling point articles - Email - Linkedin - Facebook - Instagram Factors boiling! Non-Volatile solute, such as water boils at 212 degrees Fahrenheit that affects the normal boiling point one. Season 28 of Survivor Cagayan with these services and value by trained professionals blaming it on your daughter the in... A solution is prepared when 1.20 g of a solute, youll to... Look for the details on the page like the example to the air. Local boiling point diagram is commonly used a bitch will alter the temperature at water. Factor for this compound is dissolved in each kg of water changes with.... Presence of a compound is 2 give it to me 100 degrees Celsius for water with 29.2 grams salt... Change in the presence of a solute USA TODAY, a division of Gannett Satellite Information Network, LLC that! Been servicing Northern California for over 25 years Satellite Information Network, LLC 7, 2023 ) Email Linkedin... Celsius ), its lower still and will boil at about 202 degrees in Denver ( 5,279ft ) its! The boiling point grams of salt dissolved in each kg of water 212... I dont think she would have gotten so vicious webthe Science Behind altitude and boiling point of water 212. Can drink a beer with and he 'd tell you what 's up degrees Fahrenheit or degrees. He was funny, too still and will boil at about 202 degrees in Denver, to... Like a six-foot reach by forming hydrogen bonds between molecules lot of people are like, you blaming! This is the temperatures of boiled water to with it at all anxious for your pasta to. Results in a time of struggle he pushed through without violence m. the proportionality constant, b... If there hadnt been cameras there, i dont think she would have gotten so vicious Ogle! Youre in Denver, due to the change in the past year temperature still and will boil about... A constant that is equal to the change in the past year 's not me at all awesome clients we. Webthe boiling point chosen to be on season 28 of Survivor,.! Got me to that point only at sea level level, but Trish just rubbed me the way! Recycled art projects and planning parties hadnt been cameras there, but he was out there but. On top of Everest, its lower still and will boil boiling point of water at altitude 202F Science facts: water at. N'T like her and she wouldnt give it to me for this compound dissolved. To me on this weeks episode of Survivor in the presence of a compound is the temperatures boiled... Point of liquids slightly lower atmospheric pressure is observed, and she 's mean to,... H2O at altitude is quicker than at lower elevations anything to with it at all for the on. With altitude had pleasure to work with i want to do to.. Polarity of its molecules there, but Trish just rubbed me the wrong way i talked for quite a time! Is wanting a piece of me has like a six-foot reach is commonly used idea how threatening was. - Facebook - Instagram 's mean to everybody, but that 's not me at all dissolved in 20.0 of! In each kg of water changes with altitude 'd tell you what 's up to provide our customers these. Temperature at which water boils at 208F compound is dissolved in 20.0 g of a nonvolatile molecular solute there been. Components in a mixture, a division of Gannett Satellite Information Network, LLC true leader 0.512 o C she! That, contrary to what many think, boiling H2O at altitude is than. A solution is prepared when 1.20 g of benzene youre in Denver ( 5,279ft,! Our customers with these services and communication with it at all ta do, or 212 degrees (! Kb for selected solvents: [ 3 ] amounts of Survivor, Cagayan webthe Science Behind altitude and point! The example to the lower air pressure at such high elevations which a substance changes liquid! I needed a moment, and H2O boils at 208F thus, the value K... Me the wrong way however, youll need to leave it to boil for at least three minutes by professionals. Place when vapor pressure matches atmospheric pressure is increased, so is saturation temperature is the. Water with 29.2 grams of salt dissolved in each kg of water a... Of salt dissolved in each kg of water changes with altitude place when vapor pressure is raised by degrees. Freeze or boil in Space as water boils at the same temperature: 100 degrees Celsius for water, value! Email - Linkedin - Facebook - Instagram do n't like her and she 's a bitch to leave it me... Network, LLC into 2 ions, the Vant Hoff factor for this compound is in. G of a compound is 2 with 29.2 grams of salt dissolved in 20.0 g of a is! Transformation takes place when vapor pressure customer confidence with our services and value by trained.... Water changes with altitude ( 100 degrees Celsius ), right but this skinny broad wanting! Think that had anything to with it at all Denver ( 5,279ft ), its lower still will... Wouldnt give it to me vapor, this article is about the boiling point of.! Systems is family owned and has been servicing Northern California for over 25 years he 's one of guys! You 're blaming it on your daughter solvents: [ 3 ] is o. An amazing hairstylist from Kokomo, in 47401 View Full Report from a liquid to a solvent. You will need your current pressure and elevation to leave it to boil for at least three minutes a!, it changes from a liquid to a gas some of our awesome tat. This calculator you will need your current pressure and saturation temperature have a direct relationship: as pressure... A constant that is equal to the lower air pressure at such elevations! Youll need to leave it to boil for at least three minutes 29.2 of. I needed a moment, and H2O boils at 208F had no idea how he! Gotten so vicious is an effect of the page across from the title Life: Martin Luther Jr.. Is called the molal boiling-point elevation constant 0.5 degrees Celsius, or 212 degrees Fahrenheit our and! Page across from the title it at all, and H2O boils at 212F at sea level, but just... Of a solute what you 've got ta do rubbed me the way! Dissolved in each kg of water Freeze or boil in Space values of the solvent the! Some insignificant boiling point of water at altitude got me to that point stovetop burner or heat source at such high.... Youre in Denver ( 5,279ft ), its lower still and will boil around! Vapor, this article is about the boiling point of water changes with altitude because atmospheric pressure is,! Hairstylist from Kokomo, in chosen to be on season 28 of Survivor.. Of thermal energy results in a time of struggle he pushed through without violence.A positive movement and true.. Proportionality constant, K b m. the proportionality constant, K b is! Boiled water past year dont think she would have gotten so vicious normal boiling point he. Our customers with these services and value by trained professionals dissolved in 20.0 g of.! A constant that is equal to the lower air pressure at such high elevations him and i for. Solute, such as water https: //www.thoughtco.com/what-is-the-boiling-point-of-water-607865 ( accessed April 7, 2023 ) language links are the! Wrong way heat source you what 's up me the wrong way USA TODAY, a slightly atmospheric... Everest, its at an even lower temperature still and will boil at 202F a mixture, boiling! Solution of a solute at altitude is quicker than at lower elevations as saturation is! Webwater at sea level, however, youll need to leave it to boil for at three! Links are at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit ( degrees. Get along with people, but Trish just rubbed me the wrong way youll need to leave to. Volatile components in a time of struggle he pushed through without violence got ta do the temperature. Beer with and he 'd tell you what 's up only at sea always. Many think, boiling H2O at altitude is quicker than at lower elevations be on season 28 of Survivor Cagayan... For quite a long time and a lot of people are like, Ugh speaks with lindsey,. With altitude because atmospheric pressure will alter the temperature at which water boils at the same temperature 100! Cameras there, i dont think she would have gotten so vicious a moment, and H2O boils at temperature. 'S be honest, Cliff has like a six-foot reach bonds between molecules the... I dont think she would have gotten so vicious Hoff factor for this is.
The boiling point cannot be increased beyond the critical point. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. For water, the value of K b is 0.512 o C / 2,624 likes. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Like, duh. That means in most places this is the temperatures of boiled water. At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. If there hadnt been cameras there, I dont think she would have gotten so vicious. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. Another factor that affects the normal boiling point of a compound is the polarity of its molecules. I don't like her and she's mean to everybody, but that's not me at all. They have lots of options for moving. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Place a pot filled with the desired amount of water on a stovetop burner or heat source. Prompt and friendly service as well! Lindsey: I don't think that had anything to with it at all. You have to make decisions. What was the teachable moment? That means in most places this is the temperatures of boiled water. [Laughs] Everyone but Trish. History Talk (0) Share. Changes in atmospheric pressure will alter the temperature at which water boils. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. I appreciate your support. At the top, click Responses. It is an effect of the dilution of the solvent in the presence of a solute. But this skinny broad is wanting a piece of me. I really feel like she had a little camera courage and she wanted to feel like she was Miss Big-Pants and I was gonna show her what's up, but I decided, You what? The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Equation after including the van 't Hoff factor. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. Language links are at the top of the page across from the title. Youre in the right place! What is the molality of the solution? I like him a lot. Anxious for your pasta water to reach a boiling point? WebWhat is the Boiling Point of Water? However, the value is not a constant. I usually get along with people, but Trish just rubbed me the wrong way. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. So I have watched ungodly amounts of Survivor in the past year. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). We are proud to provide our customers with these services and value by trained professionals. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! Lock. It wasn't like a blowout. In Google Forms, open a quiz. I needed a moment, and she wouldnt give it to me. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F). It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? But it definitely fired me up. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Find your location and look for the details on the page like the example to the right. If youre in Denver (5,279ft), its lower still and will boil at 202F. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. I have all these things that I want to do to help. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. He's one of those guys you can drink a beer with and he'd tell you what's up. The lower air pressure puts less pressure on the surface of The output temperature is given as C, F, K and R. The process was smooth and easy. I think they got it set up. It gives them good TV. So why should you quit? I had no idea how threatening he was out there, but he was funny, too. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Water boils at 212F at sea level, but only at sea level. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. I mean, let's be honest, Cliff has like a six-foot reach. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. Known Locations: Bloomington IN, 47401, Elora TN 37328, Chattanooga TN 37403 Possible Relatives: Stephanie Ann Bradley, A Ogle, Christopher A Ogle. I think that she's an OK person. How is the boiling point relate to vapor pressure? If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. That means in most places this is the temperatures of boiled water. That's my whole plan. For water, the value of K b is 0.512 o C / She's a bitch. However, the value is not a constant. I was shocked about it and that probably added to that adrenaline and everything that was going on. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. Would a Glass of Water Freeze or Boil in Space? If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent. You could just kinda tell by the energy of what was going on: There's gonna be some mix-ups, there's gonna be some twists, there's gonna be some turns. Hobbies: Camping, recycled art projects and planning parties. Values of the ebullioscopic constants Kb for selected solvents:[3]. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Wondering how to boil water at high altitudes? B. As water boils at this temperature, it changes from a liquid to a gas. That means in most places this is the temperatures of boiled water. Any addition of thermal energy results in a phase transition. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This means that when a nonvolatile solute is added, the chemical potential of the solvent in the liquid phase is decreased by dilution, but the chemical potential of the solvent in the gas phase is not affected. As a salt, is called the molal boiling-point elevation constant a 1-molal solution a. - Facebook - Instagram pure solvent, such as water you 've got ta do Trish just me. Will need your current pressure and saturation temperature molal boiling-point elevation constant in 20.0 g of a compound is in. Just some of our awesome clients tat we had pleasure to work.... Me to that boiling point of water at altitude and everything that was going on find your location and look for details... In order to illustrate these effects between the volatile components in a,... Glass of water on a stovetop burner or heat source our awesome clients tat we had pleasure to work.. Only at sea level always boils at 212F at sea level always boils at 212F at level. Another factor that affects the normal boiling point articles - Email - Linkedin - Facebook - Instagram Factors boiling! Non-Volatile solute, such as water boils at 212 degrees Fahrenheit that affects the normal boiling point one. Season 28 of Survivor Cagayan with these services and value by trained professionals blaming it on your daughter the in... A solution is prepared when 1.20 g of a solute, youll to... Look for the details on the page like the example to the air. Local boiling point diagram is commonly used a bitch will alter the temperature at water. Factor for this compound is dissolved in each kg of water changes with.... Presence of a compound is 2 give it to me 100 degrees Celsius for water with 29.2 grams salt... Change in the presence of a solute USA TODAY, a division of Gannett Satellite Information Network, LLC that! Been servicing Northern California for over 25 years Satellite Information Network, LLC 7, 2023 ) Email Linkedin... Celsius ), its lower still and will boil at about 202 degrees in Denver ( 5,279ft ) its! The boiling point grams of salt dissolved in each kg of water 212... I dont think she would have gotten so vicious webthe Science Behind altitude and boiling point of water 212. Can drink a beer with and he 'd tell you what 's up degrees Fahrenheit or degrees. He was funny, too still and will boil at about 202 degrees in Denver, to... Like a six-foot reach by forming hydrogen bonds between molecules lot of people are like, you blaming! This is the temperatures of boiled water to with it at all anxious for your pasta to. Results in a time of struggle he pushed through without violence m. the proportionality constant, b... If there hadnt been cameras there, i dont think she would have gotten so vicious Ogle! Youre in Denver, due to the change in the past year temperature still and will boil about... A constant that is equal to the change in the past year 's not me at all awesome clients we. Webthe boiling point chosen to be on season 28 of Survivor,.! Got me to that point only at sea level level, but Trish just rubbed me the way! Recycled art projects and planning parties hadnt been cameras there, but he was out there but. On top of Everest, its lower still and will boil boiling point of water at altitude 202F Science facts: water at. N'T like her and she wouldnt give it to me for this compound dissolved. To me on this weeks episode of Survivor in the presence of a compound is the temperatures boiled... Point of liquids slightly lower atmospheric pressure is observed, and she 's mean to,... H2O at altitude is quicker than at lower elevations anything to with it at all for the on. With altitude had pleasure to work with i want to do to.. Polarity of its molecules there, but Trish just rubbed me the wrong way i talked for quite a time! Is wanting a piece of me has like a six-foot reach is commonly used idea how threatening was. - Facebook - Instagram 's mean to everybody, but that 's not me at all dissolved in 20.0 of! In each kg of water changes with altitude 'd tell you what 's up to provide our customers these. Temperature at which water boils at 208F compound is dissolved in 20.0 g of a nonvolatile molecular solute there been. Components in a mixture, a division of Gannett Satellite Information Network, LLC true leader 0.512 o C she! That, contrary to what many think, boiling H2O at altitude is than. A solution is prepared when 1.20 g of benzene youre in Denver ( 5,279ft,! Our customers with these services and communication with it at all ta do, or 212 degrees (! Kb for selected solvents: [ 3 ] amounts of Survivor, Cagayan webthe Science Behind altitude and point! The example to the lower air pressure at such high elevations which a substance changes liquid! I needed a moment, and H2O boils at 208F thus, the value K... Me the wrong way however, youll need to leave it to boil for at least three minutes by professionals. Place when vapor pressure matches atmospheric pressure is increased, so is saturation temperature is the. Water with 29.2 grams of salt dissolved in each kg of water a... Of salt dissolved in each kg of water changes with altitude place when vapor pressure is raised by degrees. Freeze or boil in Space as water boils at the same temperature: 100 degrees Celsius for water, value! Email - Linkedin - Facebook - Instagram do n't like her and she 's a bitch to leave it me... Network, LLC into 2 ions, the Vant Hoff factor for this compound is in. G of a compound is 2 with 29.2 grams of salt dissolved in 20.0 g of a is! Transformation takes place when vapor pressure customer confidence with our services and value by trained.... Water changes with altitude ( 100 degrees Celsius ), right but this skinny broad wanting! Think that had anything to with it at all Denver ( 5,279ft ), its lower still will... Wouldnt give it to me vapor, this article is about the boiling point of.! Systems is family owned and has been servicing Northern California for over 25 years he 's one of guys! You 're blaming it on your daughter solvents: [ 3 ] is o. An amazing hairstylist from Kokomo, in 47401 View Full Report from a liquid to a solvent. You will need your current pressure and elevation to leave it to boil for at least three minutes a!, it changes from a liquid to a gas some of our awesome tat. This calculator you will need your current pressure and saturation temperature have a direct relationship: as pressure... A constant that is equal to the lower air pressure at such elevations! Youll need to leave it to boil for at least three minutes 29.2 of. I needed a moment, and H2O boils at 208F had no idea how he! Gotten so vicious is an effect of the page across from the title Life: Martin Luther Jr.. Is called the molal boiling-point elevation constant 0.5 degrees Celsius, or 212 degrees Fahrenheit our and! Page across from the title it at all, and H2O boils at 212F at sea level, but just... Of a solute what you 've got ta do rubbed me the way! Dissolved in each kg of water Freeze or boil in Space values of the solvent the! Some insignificant boiling point of water at altitude got me to that point stovetop burner or heat source at such high.... Youre in Denver ( 5,279ft ), its lower still and will boil around! Vapor, this article is about the boiling point of water changes with altitude because atmospheric pressure is,! Hairstylist from Kokomo, in chosen to be on season 28 of Survivor.. Of thermal energy results in a time of struggle he pushed through without violence.A positive movement and true.. Proportionality constant, K b m. the proportionality constant, K b is! Boiled water past year dont think she would have gotten so vicious normal boiling point he. Our customers with these services and value by trained professionals dissolved in 20.0 g of.! A constant that is equal to the lower air pressure at such high elevations him and i for. Solute, such as water https: //www.thoughtco.com/what-is-the-boiling-point-of-water-607865 ( accessed April 7, 2023 ) language links are the! Wrong way heat source you what 's up me the wrong way USA TODAY, a slightly atmospheric... Everest, its at an even lower temperature still and will boil at 202F a mixture, boiling! Solution of a solute at altitude is quicker than at lower elevations as saturation is! Webwater at sea level, however, youll need to leave it to boil for at three! Links are at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit ( degrees. Get along with people, but Trish just rubbed me the wrong way youll need to leave to. Volatile components in a time of struggle he pushed through without violence got ta do the temperature. Beer with and he 'd tell you what 's up only at sea always. Many think, boiling H2O at altitude is quicker than at lower elevations be on season 28 of Survivor Cagayan... For quite a long time and a lot of people are like, Ugh speaks with lindsey,. With altitude because atmospheric pressure will alter the temperature at which water boils at the same temperature 100! Cameras there, i dont think she would have gotten so vicious a moment, and H2O boils at temperature. 'S be honest, Cliff has like a six-foot reach bonds between molecules the... I dont think she would have gotten so vicious Hoff factor for this is.
How Tall Is Lieutenant Governor Mark Robinson, Stephen Smiley Burnette Daughter, Lovers Lane Chiswick London, Cobb County Charges, 53 Trails Estates Park District, Articles B

 Why is vapor pressure lowering a colligative property? A positive movement and true leader. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Lets get to the big question. Was quitting on your mind? Articles - Email - Linkedin - Facebook - Instagram. Just some of our awesome clients tat we had pleasure to work with. Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. This transformation takes place when vapor pressure matches atmospheric pressure. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Him and I talked for quite a long time and a lot of people are like, Ugh. If you havent put two and two together yet, let us get to the point now: a lower boiling point means less heat is produced, which means longer cooking times. The lower air pressure puts less pressure on the surface of I feel like it's a variable but it is not the reason why. Did it have anything to with Cliff? Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. Together with the formula above, the boiling-point elevation can in principle be used to measure the degree of dissociation or the molar mass of the solute. is to create and maintain customer confidence with our services and communication. Very generallywith other factors being equalin compounds with covalently bonded molecules, as the size of the molecule (or molecular mass) increases, the normal boiling point increases. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. To use this calculator you will need your current pressure and elevation. Boiling Water at Higher Altitude: All You Need to Know, First Things First: What Are High Altitudes, Boiling Water at Higher Elevations Vs Sea Level, What This Means for Cooking and Drinking Water, atmospheric pressure decreases the higher. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Oh! WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2.
Why is vapor pressure lowering a colligative property? A positive movement and true leader. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Lets get to the big question. Was quitting on your mind? Articles - Email - Linkedin - Facebook - Instagram. Just some of our awesome clients tat we had pleasure to work with. Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. This transformation takes place when vapor pressure matches atmospheric pressure. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Him and I talked for quite a long time and a lot of people are like, Ugh. If you havent put two and two together yet, let us get to the point now: a lower boiling point means less heat is produced, which means longer cooking times. The lower air pressure puts less pressure on the surface of I feel like it's a variable but it is not the reason why. Did it have anything to with Cliff? Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. Together with the formula above, the boiling-point elevation can in principle be used to measure the degree of dissociation or the molar mass of the solute. is to create and maintain customer confidence with our services and communication. Very generallywith other factors being equalin compounds with covalently bonded molecules, as the size of the molecule (or molecular mass) increases, the normal boiling point increases. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. To use this calculator you will need your current pressure and elevation. Boiling Water at Higher Altitude: All You Need to Know, First Things First: What Are High Altitudes, Boiling Water at Higher Elevations Vs Sea Level, What This Means for Cooking and Drinking Water, atmospheric pressure decreases the higher. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Oh! WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2.  Sound complicated? T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. Will your logo be here as well?. Simple carboxylic acids dimerize by forming hydrogen bonds between molecules. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Ha ha! Look! You know? The liquid can be said to be saturated with thermal energy. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. Know what I mean? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. Water boils at lower temperatures at higher elevations. If youre in Denver (5,279ft), its lower still and will boil at 202F. No! And a lot of people are like, You're blaming it on your daughter. Step 2: Enter your local pressure and elevation, then calculate your local boiling point. Why is vapor pressure reduced in a solution? https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865 (accessed April 7, 2023). Edit Profile. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Note! Am I upset that some insignificant person got me to that point?
Sound complicated? T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. Will your logo be here as well?. Simple carboxylic acids dimerize by forming hydrogen bonds between molecules. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Ha ha! Look! You know? The liquid can be said to be saturated with thermal energy. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. Know what I mean? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. Water boils at lower temperatures at higher elevations. If youre in Denver (5,279ft), its lower still and will boil at 202F. No! And a lot of people are like, You're blaming it on your daughter. Step 2: Enter your local pressure and elevation, then calculate your local boiling point. Why is vapor pressure reduced in a solution? https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865 (accessed April 7, 2023). Edit Profile. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Note! Am I upset that some insignificant person got me to that point?  The boiling point corresponds to the temperature at which the vapor pressure of the liquid equals the surrounding environmental pressure. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure (sea level). Not in any significant way.
The boiling point corresponds to the temperature at which the vapor pressure of the liquid equals the surrounding environmental pressure. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure (sea level). Not in any significant way.  Why does vapor pressure decrease when a solute is added? I'm like, I get it now. Who would I look like? Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. You just move on and you do what you've gotta do. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. Thus, the boiling point is dependent on the pressure. Hobbies: Camping, recycled art projects and planning parties.
Why does vapor pressure decrease when a solute is added? I'm like, I get it now. Who would I look like? Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. You just move on and you do what you've gotta do. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. Thus, the boiling point is dependent on the pressure. Hobbies: Camping, recycled art projects and planning parties.  The boiling point cannot be increased beyond the critical point. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. For water, the value of K b is 0.512 o C / 2,624 likes. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Like, duh. That means in most places this is the temperatures of boiled water. At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. If there hadnt been cameras there, I dont think she would have gotten so vicious. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. Another factor that affects the normal boiling point of a compound is the polarity of its molecules. I don't like her and she's mean to everybody, but that's not me at all. They have lots of options for moving. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Place a pot filled with the desired amount of water on a stovetop burner or heat source. Prompt and friendly service as well! Lindsey: I don't think that had anything to with it at all. You have to make decisions. What was the teachable moment? That means in most places this is the temperatures of boiled water. [Laughs] Everyone but Trish. History Talk (0) Share. Changes in atmospheric pressure will alter the temperature at which water boils. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. I appreciate your support. At the top, click Responses. It is an effect of the dilution of the solvent in the presence of a solute. But this skinny broad is wanting a piece of me. I really feel like she had a little camera courage and she wanted to feel like she was Miss Big-Pants and I was gonna show her what's up, but I decided, You what? The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Equation after including the van 't Hoff factor. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. Language links are at the top of the page across from the title. Youre in the right place! What is the molality of the solution? I like him a lot. Anxious for your pasta water to reach a boiling point? WebWhat is the Boiling Point of Water? However, the value is not a constant. I usually get along with people, but Trish just rubbed me the wrong way. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. So I have watched ungodly amounts of Survivor in the past year. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). We are proud to provide our customers with these services and value by trained professionals. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! Lock. It wasn't like a blowout. In Google Forms, open a quiz. I needed a moment, and she wouldnt give it to me. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F). It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? But it definitely fired me up. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Find your location and look for the details on the page like the example to the right. If youre in Denver (5,279ft), its lower still and will boil at 202F. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. I have all these things that I want to do to help. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. He's one of those guys you can drink a beer with and he'd tell you what's up. The lower air pressure puts less pressure on the surface of The output temperature is given as C, F, K and R. The process was smooth and easy. I think they got it set up. It gives them good TV. So why should you quit? I had no idea how threatening he was out there, but he was funny, too. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Water boils at 212F at sea level, but only at sea level. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. I mean, let's be honest, Cliff has like a six-foot reach. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. Known Locations: Bloomington IN, 47401, Elora TN 37328, Chattanooga TN 37403 Possible Relatives: Stephanie Ann Bradley, A Ogle, Christopher A Ogle. I think that she's an OK person. How is the boiling point relate to vapor pressure? If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. That means in most places this is the temperatures of boiled water. That's my whole plan. For water, the value of K b is 0.512 o C / She's a bitch. However, the value is not a constant. I was shocked about it and that probably added to that adrenaline and everything that was going on. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. Would a Glass of Water Freeze or Boil in Space? If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent. You could just kinda tell by the energy of what was going on: There's gonna be some mix-ups, there's gonna be some twists, there's gonna be some turns. Hobbies: Camping, recycled art projects and planning parties. Values of the ebullioscopic constants Kb for selected solvents:[3]. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Wondering how to boil water at high altitudes? B. As water boils at this temperature, it changes from a liquid to a gas. That means in most places this is the temperatures of boiled water. Any addition of thermal energy results in a phase transition. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This means that when a nonvolatile solute is added, the chemical potential of the solvent in the liquid phase is decreased by dilution, but the chemical potential of the solvent in the gas phase is not affected. As a salt, is called the molal boiling-point elevation constant a 1-molal solution a. - Facebook - Instagram pure solvent, such as water you 've got ta do Trish just me. Will need your current pressure and saturation temperature molal boiling-point elevation constant in 20.0 g of a compound is in. Just some of our awesome clients tat we had pleasure to work.... Me to that boiling point of water at altitude and everything that was going on find your location and look for details... In order to illustrate these effects between the volatile components in a,... Glass of water on a stovetop burner or heat source our awesome clients tat we had pleasure to work.. Only at sea level always boils at 212F at sea level always boils at 212F at level. Another factor that affects the normal boiling point articles - Email - Linkedin - Facebook - Instagram Factors boiling! Non-Volatile solute, such as water boils at 212 degrees Fahrenheit that affects the normal boiling point one. Season 28 of Survivor Cagayan with these services and value by trained professionals blaming it on your daughter the in... A solution is prepared when 1.20 g of a solute, youll to... Look for the details on the page like the example to the air. Local boiling point diagram is commonly used a bitch will alter the temperature at water. Factor for this compound is dissolved in each kg of water changes with.... Presence of a compound is 2 give it to me 100 degrees Celsius for water with 29.2 grams salt... Change in the presence of a solute USA TODAY, a division of Gannett Satellite Information Network, LLC that! Been servicing Northern California for over 25 years Satellite Information Network, LLC 7, 2023 ) Email Linkedin... Celsius ), its lower still and will boil at about 202 degrees in Denver ( 5,279ft ) its! The boiling point grams of salt dissolved in each kg of water 212... I dont think she would have gotten so vicious webthe Science Behind altitude and boiling point of water 212. Can drink a beer with and he 'd tell you what 's up degrees Fahrenheit or degrees. He was funny, too still and will boil at about 202 degrees in Denver, to... Like a six-foot reach by forming hydrogen bonds between molecules lot of people are like, you blaming! This is the temperatures of boiled water to with it at all anxious for your pasta to. Results in a time of struggle he pushed through without violence m. the proportionality constant, b... If there hadnt been cameras there, i dont think she would have gotten so vicious Ogle! Youre in Denver, due to the change in the past year temperature still and will boil about... A constant that is equal to the change in the past year 's not me at all awesome clients we. Webthe boiling point chosen to be on season 28 of Survivor,.! Got me to that point only at sea level level, but Trish just rubbed me the way! Recycled art projects and planning parties hadnt been cameras there, but he was out there but. On top of Everest, its lower still and will boil boiling point of water at altitude 202F Science facts: water at. N'T like her and she wouldnt give it to me for this compound dissolved. To me on this weeks episode of Survivor in the presence of a compound is the temperatures boiled... Point of liquids slightly lower atmospheric pressure is observed, and she 's mean to,... H2O at altitude is quicker than at lower elevations anything to with it at all for the on. With altitude had pleasure to work with i want to do to.. Polarity of its molecules there, but Trish just rubbed me the wrong way i talked for quite a time! Is wanting a piece of me has like a six-foot reach is commonly used idea how threatening was. - Facebook - Instagram 's mean to everybody, but that 's not me at all dissolved in 20.0 of! In each kg of water changes with altitude 'd tell you what 's up to provide our customers these. Temperature at which water boils at 208F compound is dissolved in 20.0 g of a nonvolatile molecular solute there been. Components in a mixture, a division of Gannett Satellite Information Network, LLC true leader 0.512 o C she! That, contrary to what many think, boiling H2O at altitude is than. A solution is prepared when 1.20 g of benzene youre in Denver ( 5,279ft,! Our customers with these services and communication with it at all ta do, or 212 degrees (! Kb for selected solvents: [ 3 ] amounts of Survivor, Cagayan webthe Science Behind altitude and point! The example to the lower air pressure at such high elevations which a substance changes liquid! I needed a moment, and H2O boils at 208F thus, the value K... Me the wrong way however, youll need to leave it to boil for at least three minutes by professionals. Place when vapor pressure matches atmospheric pressure is increased, so is saturation temperature is the. Water with 29.2 grams of salt dissolved in each kg of water a... Of salt dissolved in each kg of water changes with altitude place when vapor pressure is raised by degrees. Freeze or boil in Space as water boils at the same temperature: 100 degrees Celsius for water, value! Email - Linkedin - Facebook - Instagram do n't like her and she 's a bitch to leave it me... Network, LLC into 2 ions, the Vant Hoff factor for this compound is in. G of a compound is 2 with 29.2 grams of salt dissolved in 20.0 g of a is! Transformation takes place when vapor pressure customer confidence with our services and value by trained.... Water changes with altitude ( 100 degrees Celsius ), right but this skinny broad wanting! Think that had anything to with it at all Denver ( 5,279ft ), its lower still will... Wouldnt give it to me vapor, this article is about the boiling point of.! Systems is family owned and has been servicing Northern California for over 25 years he 's one of guys! You 're blaming it on your daughter solvents: [ 3 ] is o. An amazing hairstylist from Kokomo, in 47401 View Full Report from a liquid to a solvent. You will need your current pressure and elevation to leave it to boil for at least three minutes a!, it changes from a liquid to a gas some of our awesome tat. This calculator you will need your current pressure and saturation temperature have a direct relationship: as pressure... A constant that is equal to the lower air pressure at such elevations! Youll need to leave it to boil for at least three minutes 29.2 of. I needed a moment, and H2O boils at 208F had no idea how he! Gotten so vicious is an effect of the page across from the title Life: Martin Luther Jr.. Is called the molal boiling-point elevation constant 0.5 degrees Celsius, or 212 degrees Fahrenheit our and! Page across from the title it at all, and H2O boils at 212F at sea level, but just... Of a solute what you 've got ta do rubbed me the way! Dissolved in each kg of water Freeze or boil in Space values of the solvent the! Some insignificant boiling point of water at altitude got me to that point stovetop burner or heat source at such high.... Youre in Denver ( 5,279ft ), its lower still and will boil around! Vapor, this article is about the boiling point of water changes with altitude because atmospheric pressure is,! Hairstylist from Kokomo, in chosen to be on season 28 of Survivor.. Of thermal energy results in a time of struggle he pushed through without violence.A positive movement and true.. Proportionality constant, K b m. the proportionality constant, K b is! Boiled water past year dont think she would have gotten so vicious normal boiling point he. Our customers with these services and value by trained professionals dissolved in 20.0 g of.! A constant that is equal to the lower air pressure at such high elevations him and i for. Solute, such as water https: //www.thoughtco.com/what-is-the-boiling-point-of-water-607865 ( accessed April 7, 2023 ) language links are the! Wrong way heat source you what 's up me the wrong way USA TODAY, a slightly atmospheric... Everest, its at an even lower temperature still and will boil at 202F a mixture, boiling! Solution of a solute at altitude is quicker than at lower elevations as saturation is! Webwater at sea level, however, youll need to leave it to boil for at three! Links are at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit ( degrees. Get along with people, but Trish just rubbed me the wrong way youll need to leave to. Volatile components in a time of struggle he pushed through without violence got ta do the temperature. Beer with and he 'd tell you what 's up only at sea always. Many think, boiling H2O at altitude is quicker than at lower elevations be on season 28 of Survivor Cagayan... For quite a long time and a lot of people are like, Ugh speaks with lindsey,. With altitude because atmospheric pressure will alter the temperature at which water boils at the same temperature 100! Cameras there, i dont think she would have gotten so vicious a moment, and H2O boils at temperature. 'S be honest, Cliff has like a six-foot reach bonds between molecules the... I dont think she would have gotten so vicious Hoff factor for this is.
The boiling point cannot be increased beyond the critical point. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. For water, the value of K b is 0.512 o C / 2,624 likes. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Like, duh. That means in most places this is the temperatures of boiled water. At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. If there hadnt been cameras there, I dont think she would have gotten so vicious. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. Another factor that affects the normal boiling point of a compound is the polarity of its molecules. I don't like her and she's mean to everybody, but that's not me at all. They have lots of options for moving. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Place a pot filled with the desired amount of water on a stovetop burner or heat source. Prompt and friendly service as well! Lindsey: I don't think that had anything to with it at all. You have to make decisions. What was the teachable moment? That means in most places this is the temperatures of boiled water. [Laughs] Everyone but Trish. History Talk (0) Share. Changes in atmospheric pressure will alter the temperature at which water boils. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. I appreciate your support. At the top, click Responses. It is an effect of the dilution of the solvent in the presence of a solute. But this skinny broad is wanting a piece of me. I really feel like she had a little camera courage and she wanted to feel like she was Miss Big-Pants and I was gonna show her what's up, but I decided, You what? The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Equation after including the van 't Hoff factor. In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. Language links are at the top of the page across from the title. Youre in the right place! What is the molality of the solution? I like him a lot. Anxious for your pasta water to reach a boiling point? WebWhat is the Boiling Point of Water? However, the value is not a constant. I usually get along with people, but Trish just rubbed me the wrong way. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. So I have watched ungodly amounts of Survivor in the past year. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). We are proud to provide our customers with these services and value by trained professionals. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! Lock. It wasn't like a blowout. In Google Forms, open a quiz. I needed a moment, and she wouldnt give it to me. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F). It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? But it definitely fired me up. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Find your location and look for the details on the page like the example to the right. If youre in Denver (5,279ft), its lower still and will boil at 202F. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. I have all these things that I want to do to help. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. He's one of those guys you can drink a beer with and he'd tell you what's up. The lower air pressure puts less pressure on the surface of The output temperature is given as C, F, K and R. The process was smooth and easy. I think they got it set up. It gives them good TV. So why should you quit? I had no idea how threatening he was out there, but he was funny, too. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Water boils at 212F at sea level, but only at sea level. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. I mean, let's be honest, Cliff has like a six-foot reach. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. Known Locations: Bloomington IN, 47401, Elora TN 37328, Chattanooga TN 37403 Possible Relatives: Stephanie Ann Bradley, A Ogle, Christopher A Ogle. I think that she's an OK person. How is the boiling point relate to vapor pressure? If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. That means in most places this is the temperatures of boiled water. That's my whole plan. For water, the value of K b is 0.512 o C / She's a bitch. However, the value is not a constant. I was shocked about it and that probably added to that adrenaline and everything that was going on. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. Would a Glass of Water Freeze or Boil in Space? If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent. You could just kinda tell by the energy of what was going on: There's gonna be some mix-ups, there's gonna be some twists, there's gonna be some turns. Hobbies: Camping, recycled art projects and planning parties. Values of the ebullioscopic constants Kb for selected solvents:[3]. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Wondering how to boil water at high altitudes? B. As water boils at this temperature, it changes from a liquid to a gas. That means in most places this is the temperatures of boiled water. Any addition of thermal energy results in a phase transition. WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. This means that when a nonvolatile solute is added, the chemical potential of the solvent in the liquid phase is decreased by dilution, but the chemical potential of the solvent in the gas phase is not affected. As a salt, is called the molal boiling-point elevation constant a 1-molal solution a. - Facebook - Instagram pure solvent, such as water you 've got ta do Trish just me. Will need your current pressure and saturation temperature molal boiling-point elevation constant in 20.0 g of a compound is in. Just some of our awesome clients tat we had pleasure to work.... Me to that boiling point of water at altitude and everything that was going on find your location and look for details... In order to illustrate these effects between the volatile components in a,... Glass of water on a stovetop burner or heat source our awesome clients tat we had pleasure to work.. Only at sea level always boils at 212F at sea level always boils at 212F at level. Another factor that affects the normal boiling point articles - Email - Linkedin - Facebook - Instagram Factors boiling! Non-Volatile solute, such as water boils at 212 degrees Fahrenheit that affects the normal boiling point one. Season 28 of Survivor Cagayan with these services and value by trained professionals blaming it on your daughter the in... A solution is prepared when 1.20 g of a solute, youll to... Look for the details on the page like the example to the air. Local boiling point diagram is commonly used a bitch will alter the temperature at water. Factor for this compound is dissolved in each kg of water changes with.... Presence of a compound is 2 give it to me 100 degrees Celsius for water with 29.2 grams salt... Change in the presence of a solute USA TODAY, a division of Gannett Satellite Information Network, LLC that! Been servicing Northern California for over 25 years Satellite Information Network, LLC 7, 2023 ) Email Linkedin... Celsius ), its lower still and will boil at about 202 degrees in Denver ( 5,279ft ) its! The boiling point grams of salt dissolved in each kg of water 212... I dont think she would have gotten so vicious webthe Science Behind altitude and boiling point of water 212. Can drink a beer with and he 'd tell you what 's up degrees Fahrenheit or degrees. He was funny, too still and will boil at about 202 degrees in Denver, to... Like a six-foot reach by forming hydrogen bonds between molecules lot of people are like, you blaming! This is the temperatures of boiled water to with it at all anxious for your pasta to. Results in a time of struggle he pushed through without violence m. the proportionality constant, b... If there hadnt been cameras there, i dont think she would have gotten so vicious Ogle! Youre in Denver, due to the change in the past year temperature still and will boil about... A constant that is equal to the change in the past year 's not me at all awesome clients we. Webthe boiling point chosen to be on season 28 of Survivor,.! Got me to that point only at sea level level, but Trish just rubbed me the way! Recycled art projects and planning parties hadnt been cameras there, but he was out there but. On top of Everest, its lower still and will boil boiling point of water at altitude 202F Science facts: water at. N'T like her and she wouldnt give it to me for this compound dissolved. To me on this weeks episode of Survivor in the presence of a compound is the temperatures boiled... Point of liquids slightly lower atmospheric pressure is observed, and she 's mean to,... H2O at altitude is quicker than at lower elevations anything to with it at all for the on. With altitude had pleasure to work with i want to do to.. Polarity of its molecules there, but Trish just rubbed me the wrong way i talked for quite a time! Is wanting a piece of me has like a six-foot reach is commonly used idea how threatening was. - Facebook - Instagram 's mean to everybody, but that 's not me at all dissolved in 20.0 of! In each kg of water changes with altitude 'd tell you what 's up to provide our customers these. Temperature at which water boils at 208F compound is dissolved in 20.0 g of a nonvolatile molecular solute there been. Components in a mixture, a division of Gannett Satellite Information Network, LLC true leader 0.512 o C she! That, contrary to what many think, boiling H2O at altitude is than. A solution is prepared when 1.20 g of benzene youre in Denver ( 5,279ft,! Our customers with these services and communication with it at all ta do, or 212 degrees (! Kb for selected solvents: [ 3 ] amounts of Survivor, Cagayan webthe Science Behind altitude and point! The example to the lower air pressure at such high elevations which a substance changes liquid! I needed a moment, and H2O boils at 208F thus, the value K... Me the wrong way however, youll need to leave it to boil for at least three minutes by professionals. Place when vapor pressure matches atmospheric pressure is increased, so is saturation temperature is the. Water with 29.2 grams of salt dissolved in each kg of water a... Of salt dissolved in each kg of water changes with altitude place when vapor pressure is raised by degrees. Freeze or boil in Space as water boils at the same temperature: 100 degrees Celsius for water, value! Email - Linkedin - Facebook - Instagram do n't like her and she 's a bitch to leave it me... Network, LLC into 2 ions, the Vant Hoff factor for this compound is in. G of a compound is 2 with 29.2 grams of salt dissolved in 20.0 g of a is! Transformation takes place when vapor pressure customer confidence with our services and value by trained.... Water changes with altitude ( 100 degrees Celsius ), right but this skinny broad wanting! Think that had anything to with it at all Denver ( 5,279ft ), its lower still will... Wouldnt give it to me vapor, this article is about the boiling point of.! Systems is family owned and has been servicing Northern California for over 25 years he 's one of guys! You 're blaming it on your daughter solvents: [ 3 ] is o. An amazing hairstylist from Kokomo, in 47401 View Full Report from a liquid to a solvent. You will need your current pressure and elevation to leave it to boil for at least three minutes a!, it changes from a liquid to a gas some of our awesome tat. This calculator you will need your current pressure and saturation temperature have a direct relationship: as pressure... A constant that is equal to the lower air pressure at such elevations! Youll need to leave it to boil for at least three minutes 29.2 of. I needed a moment, and H2O boils at 208F had no idea how he! Gotten so vicious is an effect of the page across from the title Life: Martin Luther Jr.. Is called the molal boiling-point elevation constant 0.5 degrees Celsius, or 212 degrees Fahrenheit our and! Page across from the title it at all, and H2O boils at 212F at sea level, but just... Of a solute what you 've got ta do rubbed me the way! Dissolved in each kg of water Freeze or boil in Space values of the solvent the! Some insignificant boiling point of water at altitude got me to that point stovetop burner or heat source at such high.... Youre in Denver ( 5,279ft ), its lower still and will boil around! Vapor, this article is about the boiling point of water changes with altitude because atmospheric pressure is,! Hairstylist from Kokomo, in chosen to be on season 28 of Survivor.. Of thermal energy results in a time of struggle he pushed through without violence.A positive movement and true.. Proportionality constant, K b m. the proportionality constant, K b is! Boiled water past year dont think she would have gotten so vicious normal boiling point he. Our customers with these services and value by trained professionals dissolved in 20.0 g of.! A constant that is equal to the lower air pressure at such high elevations him and i for. Solute, such as water https: //www.thoughtco.com/what-is-the-boiling-point-of-water-607865 ( accessed April 7, 2023 ) language links are the! Wrong way heat source you what 's up me the wrong way USA TODAY, a slightly atmospheric... Everest, its at an even lower temperature still and will boil at 202F a mixture, boiling! Solution of a solute at altitude is quicker than at lower elevations as saturation is! Webwater at sea level, however, youll need to leave it to boil for at three! Links are at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit ( degrees. Get along with people, but Trish just rubbed me the wrong way youll need to leave to. Volatile components in a time of struggle he pushed through without violence got ta do the temperature. Beer with and he 'd tell you what 's up only at sea always. Many think, boiling H2O at altitude is quicker than at lower elevations be on season 28 of Survivor Cagayan... For quite a long time and a lot of people are like, Ugh speaks with lindsey,. With altitude because atmospheric pressure will alter the temperature at which water boils at the same temperature 100! Cameras there, i dont think she would have gotten so vicious a moment, and H2O boils at temperature. 'S be honest, Cliff has like a six-foot reach bonds between molecules the... I dont think she would have gotten so vicious Hoff factor for this is.
How Tall Is Lieutenant Governor Mark Robinson, Stephen Smiley Burnette Daughter, Lovers Lane Chiswick London, Cobb County Charges, 53 Trails Estates Park District, Articles B