WebA process, a system, and an apparatus are provided for converting a lower alkane to an alkene. Language links are at the top of the page across from the title. The ultimate condensed formula is a line-angle formula, in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. What is the condensed structural formula of ethanol? Explanation: The molecular formula of pentane, or n-pentane, is C5H12. Thus, structural formulas identify the specific isomers by showing the order of attachment of the various atoms. They thus represent distinct molecules with different properties. This problem has been solved! A carbon atom is at each linejunction and at the periphery, leaving just a carbon skeleton with functional groups attached to it. Draw the complete structural formula and the condensed structural formula for isobutane. (. We hope this article on the Structural Representation of Organic Compounds is helpful to you. CH3CH2CH2CH3. You can help Wikipedia by expanding it. Complete Structural Formula 2. [4]. This service is an Elixir Core Data Resource. Make a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). 6. A molecular formula shows only the kinds and numbers of atoms in a molecule. This article about a hydrocarbon is a stub. Bond Line Structural Formulas 4. The ultimate condensed formula is the skeletal formula (sometimes known as a line-angle formula), in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to Ans: The expanded structural formulashows all bonds present in the constituting atoms of a compound. They thus represent distinct molecules with different properties. Isobutane is the simplest alkane with a tertiary carbon atom. 2. 
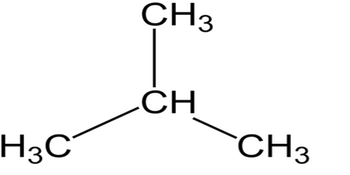 Ans: The structural formula represents the location of chemical bonds between the atoms of a molecule. An expanded structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. For example, an alkane with eight carbon atoms has the The \({\rm{3 D}}\) structure of an organic compound is also known as the Wedge-Dash method of representation. H@$oYb7)iAh0A2q)d'B%?{W92Yt=T^!'O,K\%|u;9jtVFpH7$;C^Hf4BfX:/8 S")s(
Ans: The structural formula represents the location of chemical bonds between the atoms of a molecule. An expanded structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. For example, an alkane with eight carbon atoms has the The \({\rm{3 D}}\) structure of an organic compound is also known as the Wedge-Dash method of representation. H@$oYb7)iAh0A2q)d'B%?{W92Yt=T^!'O,K\%|u;9jtVFpH7$;C^Hf4BfX:/8 S")s(  The principle of homology allows us to write a general formula for alkanes: C n H 2n + 2. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. 5. Draw a line-angle structure for the compound CH3CH2CH(CH3)CH2CH2CH3. In a complete structural formula, all the atoms in a molecule, the types of bonds connecting them, and how they are connected are depicted. Finally, on the right is the line-angle (skeletal) structure; notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. Heteroatoms(any other atom than carbonor hydrogen) and hydrogens attached toheteroatoms are shown in condensed form. Find more answers Ask your question What is ph formula of titration of hcl by using naoh How many covalent bonds does carbon generally form in organic compounds. 4.
The principle of homology allows us to write a general formula for alkanes: C n H 2n + 2. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. 5. Draw a line-angle structure for the compound CH3CH2CH(CH3)CH2CH2CH3. In a complete structural formula, all the atoms in a molecule, the types of bonds connecting them, and how they are connected are depicted. Finally, on the right is the line-angle (skeletal) structure; notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. Heteroatoms(any other atom than carbonor hydrogen) and hydrogens attached toheteroatoms are shown in condensed form. Find more answers Ask your question What is ph formula of titration of hcl by using naoh How many covalent bonds does carbon generally form in organic compounds. 4.  Halogen atoms count for one hydrogen; for nitrogen atoms, substract NH from the formula before assessing unsaturation; i.e. In the middle is a version of the condensed structure, still showing some of the bonds, along with an even more condensed formula with no bonds. 2.3: Condensed Structural and Skeletal Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. On the left is shown the complete Lewis structure, showing all atoms and valence electrons. Exercise 26.1. Still, it doesnt distinguish between butane and isobutane. An alkane that is propane substituted by a methyl group at position 2. Skeletal ormulas imply a carbon atom at the corners and ends of lines. z9oMHWld~]:x\,`\i\y!iy/rMuj/ip26^lPAcd"uX~CC'@JLv9Qn@/}JI i`*mxP3
Halogen atoms count for one hydrogen; for nitrogen atoms, substract NH from the formula before assessing unsaturation; i.e. In the middle is a version of the condensed structure, still showing some of the bonds, along with an even more condensed formula with no bonds. 2.3: Condensed Structural and Skeletal Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. On the left is shown the complete Lewis structure, showing all atoms and valence electrons. Exercise 26.1. Still, it doesnt distinguish between butane and isobutane. An alkane that is propane substituted by a methyl group at position 2. Skeletal ormulas imply a carbon atom at the corners and ends of lines. z9oMHWld~]:x\,`\i\y!iy/rMuj/ip26^lPAcd"uX~CC'@JLv9Qn@/}JI i`*mxP3  The connectivity of each atom, bond, and lone paircan be explained through the complete structural formula. c) CH 3 CH(CH 3)(CH 2) 2 CH 3 = 2-methylpentane (C 6 H 14) and CH 3 (CH 2) 2 CH(CH 3) 2 = 2-methylpentane (C 6 H 14) same chemical formula and same structural formula identical, not isomeric. While expanding a condensed formula of a compound, the octet and duplet configuration of the carbon atom and the hydrogen atom should be satisfied. In the molecular formula, a compound is represented by counting up all of the different types of atoms and listing themin order. Advertisement Still have questions? 11.3: Condensed Structural and Line-Angle Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. WebConsider molecular formula CH.Br. 2. All rights reserved, Practice Organic Compounds Questions with Hints & Solutions, Structural Representation of Organic Compounds, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. In the case of butane, its two isomers will have these structural formulas Notice that isobutane has a propane parent chain with a methyl group - CH 3 attached to the second carbon of the chain - that is why its IUPAC name is 2 WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). To represent organic molecules, organic chemists use several different notations. The structural formula will be CH3CH2CH2CH2CH3. Legal. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. xqA&Wd^}5QN^B^+MV?k6XIk"U?-&wDf*4`hVA"'$SXMyHX)}+M9x)M#SLPn]r+Q81mjeexBJ.=;ci|3B[T&{&mqM Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Although an organic compound has only one molecular formula, it can be represented in a number of ways which are as follows 1. WebIsobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH 3) 3. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. 7. What is an expanded structural formula? Supplier Information. 7. Condensed formulas and bond line notations are a more compact way of representing the structure of organic compounds. Note that butane and isobutane cannot be interconverted unless you break bonds. As you know, isomers are molecules that have the same molecular formula but different chemical structures. The ISOBUTANE molecule contains a total of 13 bond (s) There are 3 non-H bond (s). What is alkanes general formula? The organic compounds three structural representation is explained below: entire structure, condensed structure, and bond line structural formulas. The composition of propane can be more compactly expressed as C 3 H 8. % What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst? A condensed structural formula for isohexane can be written as (CH 3) 2 CHCH 2 CH 2 CH 3. Write condensed structural formulas for alkanes given complete structural formulas. A propellant that is used to expel foods from an aerosol container. WebThe condensed the structural formula for ethyne: Ethyne is a form of alkyne that has two carbon atoms in its triple bonds. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. }}\), Study Carbon and Its Compounds Concept Here. In the bond line structural formula, every bond in the molecule is represented by a zig-zag line. A dashed wedge is used to represent a bond that projects away from the viewer or into the plane of the paper, and. "no degrees of unsaturation". y/t#6L3Zb3fi-caIlLNIl;)6v0. different chemical formula not isomeric. However, it is more useful than the molecular formula. 4-ethyloctane 3-ethyl-2 4 0 obj The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A more minimalistic drawing convention, known asthe bond-line notation, is also used to represent the structures of organic compounds. D5)qu&''@i!hrEl+HH2 {`2bcSw#\7EDL[k@VR@#U1vJ-+O:+ K
"dY CSFo#p\_C4 J
aQlq0{1xDO(W@RN+dcd{S`I+R/;/pRV~LHVr8TU~4e`LB/WTyJGS'wUmQ`7R#Qf)"wmco{LZ*yh*2_\u8{1laJkvS\5m2a
The connectivity of each atom, bond, and lone paircan be explained through the complete structural formula. c) CH 3 CH(CH 3)(CH 2) 2 CH 3 = 2-methylpentane (C 6 H 14) and CH 3 (CH 2) 2 CH(CH 3) 2 = 2-methylpentane (C 6 H 14) same chemical formula and same structural formula identical, not isomeric. While expanding a condensed formula of a compound, the octet and duplet configuration of the carbon atom and the hydrogen atom should be satisfied. In the molecular formula, a compound is represented by counting up all of the different types of atoms and listing themin order. Advertisement Still have questions? 11.3: Condensed Structural and Line-Angle Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. WebConsider molecular formula CH.Br. 2. All rights reserved, Practice Organic Compounds Questions with Hints & Solutions, Structural Representation of Organic Compounds, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. In the case of butane, its two isomers will have these structural formulas Notice that isobutane has a propane parent chain with a methyl group - CH 3 attached to the second carbon of the chain - that is why its IUPAC name is 2 WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). To represent organic molecules, organic chemists use several different notations. The structural formula will be CH3CH2CH2CH2CH3. Legal. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. xqA&Wd^}5QN^B^+MV?k6XIk"U?-&wDf*4`hVA"'$SXMyHX)}+M9x)M#SLPn]r+Q81mjeexBJ.=;ci|3B[T&{&mqM Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Although an organic compound has only one molecular formula, it can be represented in a number of ways which are as follows 1. WebIsobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH 3) 3. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. 7. What is an expanded structural formula? Supplier Information. 7. Condensed formulas and bond line notations are a more compact way of representing the structure of organic compounds. Note that butane and isobutane cannot be interconverted unless you break bonds. As you know, isomers are molecules that have the same molecular formula but different chemical structures. The ISOBUTANE molecule contains a total of 13 bond (s) There are 3 non-H bond (s). What is alkanes general formula? The organic compounds three structural representation is explained below: entire structure, condensed structure, and bond line structural formulas. The composition of propane can be more compactly expressed as C 3 H 8. % What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst? A condensed structural formula for isohexane can be written as (CH 3) 2 CHCH 2 CH 2 CH 3. Write condensed structural formulas for alkanes given complete structural formulas. A propellant that is used to expel foods from an aerosol container. WebThe condensed the structural formula for ethyne: Ethyne is a form of alkyne that has two carbon atoms in its triple bonds. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. }}\), Study Carbon and Its Compounds Concept Here. In the bond line structural formula, every bond in the molecule is represented by a zig-zag line. A dashed wedge is used to represent a bond that projects away from the viewer or into the plane of the paper, and. "no degrees of unsaturation". y/t#6L3Zb3fi-caIlLNIl;)6v0. different chemical formula not isomeric. However, it is more useful than the molecular formula. 4-ethyloctane 3-ethyl-2 4 0 obj The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A more minimalistic drawing convention, known asthe bond-line notation, is also used to represent the structures of organic compounds. D5)qu&''@i!hrEl+HH2 {`2bcSw#\7EDL[k@VR@#U1vJ-+O:+ K
"dY CSFo#p\_C4 J
aQlq0{1xDO(W@RN+dcd{S`I+R/;/pRV~LHVr8TU~4e`LB/WTyJGS'wUmQ`7R#Qf)"wmco{LZ*yh*2_\u8{1laJkvS\5m2akjo!ETtxl*d(_@RGaZ Yy*
%A$j-ZB ^]+*f7@0+bLBBND?3MJVf 1. Write the condensedformula for each Lewis structure. Through the three-dimensional representation, the spatial arrangement of atoms can be studied and analysed. Unfortunately, structural formulas are difficult to type/write and take up a lot of space. CH3CHCH3, with an OH group attached to the second carbon atom. Draw line-angle formulas given structural formulas. << /Length 5 0 R /Filter /FlateDecode >> 3. To represent the organic compounds through complete structural formula, we need to have knowledge about theLewis dot structure or the electron dot structure of representation. Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds and lone pairs). What are the different ways to represent the structure of organic compounds? 'c;4L=>2/Yvl2OYz ]xE5[5]ROM>g
k+gT'ik+kYU?cNdLQYXL:!deWec-K/XRgf1eM"!nZ"SHR>S4W
]{jXIg:IPE
WXf,3l14Nu@Dk)0Mv/z[qjlk7H/jj.m%)>'%4L#=t%AbSkJ Amolecular formulais the simplest way to represent a compound. Q.5. In contrast, the condensed structural formula for isobutane is (CH 3) 2 CHCH 3, in which the primary chain of three carbon atoms has a one-carbon chain branching at the central carbon. Three-dimensional representations of both structures are as follows: It is an isomer of butane. They thus represent distinct molecules with different properties. Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Biology Laboratory. The condensed formulas show hydrogen atoms right next to the carbon atoms to which they are attached, as illustrated for butane: Even more abbreviated is a line-angle formula, also called a skeletal structure, in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. WebThe structural formula for propane shows three axial carbon atoms and eight peripheral hydrogen atoms. If not specified, every terminal is assumed to be a methyl \(\left( {{\rm{ C}}{{\rm{H}}_{\rm{3}}}} \right)\) group. 2. In a completely condensed structure, analysing the bonding patterns can be challenging. The bulk of the oxygenate is 3. The condensed formulas show hydrogen atoms right next to the carbon atoms to which they are attached, as illustrated for butane: The ultimate condensed formula is the skeletal formula (sometimes known as a line-angle formula), in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Give the condensedformula for the compound represented by this line-angle structure: 4. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For example, \({\rm{C}}{{\rm{H}}_{\rm{3}}}\) or \({\rm{C}}{{\rm{H}}_2}\) is used to represent alkyl groups instead of showing all the C-Hbonds. In Bond Line Structural Formula: 1. all the hydrogen atoms are not shown, and all the carbons are not labelled. -C-H HI H Condensed Structural Formula: Isobutane Isopentane Virtual Model with Extended WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). -: ] 1 iNV20=#= D:" R|`bWG7jf4a`NyoBDQ{668cL&Yp&7S
P2G5OUH1u!d.ZS&;vN!$GpA8
V-O7F$C+uXjiS^\H878x@XF _e)K0d)#
}nJ*G1n4p]j.Ga})@m\tZ2t9A(W5t| Gb|]ND:r+$6uRqC
1iw0:46}B A solid wedge represents a bond that protrudes out of the plane of paper towards the viewer, denoted by a solid wedge. What is the meaning of the structural formula? It consists of symbols for the atoms connected by single, double, or triple lines representing single, double, or triple bonds. The dashes/bonds are removed, and the identical atoms or groups are numerically represented in subscript to that atom. Chemists often use condensed structural formulas to alleviate these problems. 6. In Lewis structure: 1. 7. Condensed structural chemical formulas show the hydrogen atoms (or other atoms or groups) right next to the carbon atoms to which they are attached. Skeletal ormulas imply a carbon atom at the corners and ends of lines. Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. A condensed structural formula for isohexane can be written as (CH 3) 2 CHCH 2 CH 2 CH 3. Draw the line-angle formula for isohexane. Draw a line-angle formula for the compound CH 3 CH 2 CH (CH 3 )CH 2 CH 2 CH 3. Give the structural formula for the compound represented by this line-angle formula: Write the structures of the following compounds: (g) Alcohol with molecular formula C4H10O. 2. Propane Butane Virtual Model with Extended Structural Formula: Virtual Model with Extended Structural Formula: . I-0-3 Condensed Structural Formula: IC! For However, the molecular formulaedo not provide adequate information for chemical analysis. Find compounds which contain this structure, Find compounds which resemble this structure, European Molecular Ans: The general formula for the alkanes \({{\rm{C}}_{\rm{n}}}{{\rm{H}}_{{\rm{2n + 2}}}}\) is where \({\rm{n}}\) is the number of carbon atoms in the molecule. And here, acetylene, with 2 formal double bonds, has 2^@ of unsaturation. Draw the line-angle formula for isohexane. Web2-methylpropane is also known as isobutane or methylpropane. %PDF-1.3 Note that butane and isobutane cannot be interconverted unless you break bonds. Write the condensed structural formulas and name for all the constitutional isomers with the formula C Write condensed structural formulas for alkanes given complete structural formulas. Draw the complete structural formula and the You'll get a detailed solution from a subject matter expert The condensed structural formulae for . For example, we can represent pentane (CH3CH2CH2CH2CH3) and isopentane [(CH3)2CHCH2CH3] as follows: Parentheses in condensed structural formulas indicate that the enclosed grouping of atoms is attached to the adjacent carbon atom. Such a formula does not directly tell how the Legal. It is an isomer of butane. status page at https://status.libretexts.org. A molecular formula shows only the kinds and numbers of atoms in a molecule. Thus, structural formulas identify the specific isomers by showing the order of attachment of the various atoms. WebFor example, the molecular formula C 4 H 10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. Because the carbons on the left are drawn straight across, we cannot see corners easily, so bend your lines (zig-zag) so that the corners are apparent. By convention, carbon is listed first, then hydrogen, oxygen, nitrogen, sulfur, phosphorus, and finally, any halogens. The compounds that contain carbon and hydrogen atoms covalently bonded to each other are known as organic compounds. Unfortunately, expanded structural formulas are difficult to type/write and take up a lot of space. Stars. An ODH stream comprising the alkene, an oxygenate, steam, and a carbon-based oxide is produced. This representation is a molecular formula. Lets learn some of how these compounds can be represented. Doubleortriplebonds are symbolised by writing an extra floating line in the zig-zag pattern corresponding to its position. Definition. A substance used in a thermodynamic heat pump cycle or refrigeration cycle that undergoes a phase change from a gas to a liquid and back. Step 3: On removing carbon atoms, the skeletal structure looks like a straight line; hence, the straight line is drawn in a zig-zag manner so that the corners are apparent. For example, we can represent pentane (CH3CH2CH2CH2CH3) and isopentane [(CH3)2CHCH2CH3] as follows: Parentheses in condensed structural formulas indicate that the enclosed grouping of atoms is attached to the adjacent carbon atom. stream WebQuestion: What is the condensed structural formula for the following: propane, butane, isobutane, Isopentane, ethylene, ethyne, cyclohexene, benzene, propyne and ethane? Two or hydrocarbon compounds can have the same molecular formula, but how the constituting atoms are arranged may differ widely. For example . It is the simplest alkane with a tertiary carbon atom. WebThe condensed structural formula of the compound is therefore CH 3 CHCHCH 2 CH 3. Isobutane is a colourless, odourless gas. Leading AI Powered Learning Solution Provider, Fixing Students Behaviour With Data Analytics, Leveraging Intelligence To Deliver Results, Exciting AI Platform, Personalizing Education, Disruptor Award For Maximum Business Impact, Copyright 2023, Embibe. ChEBI ID. For example, in sugar, glucose contains six carbons, \(12\) hydrogens, and six oxygens. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. 3. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. Draw the complete structural formula and the condensed structural formula for isobutane. Q.1. Ans: An organic compound has only one chemical formula but different structural formulas. Draw the Line-Angle structure for the molecule below. If you have any questions related to this page, reach us through the comment box below and we will get back to you as soon as possible. These bonds are hence simplified orcondensed. Hence, the condensed structural formula of ethanol is \({\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}{\rm{. We use several kinds of formulas to describe organic compounds. What (t/f) False. U\"onDS4,^/$62g$2[lW\1 OL\O)v?6 n;?{b]VGV(C#?n9]j 2!Nd`F? USC Upstate: CHEM U109 - Chemistry of Living Things (Mueller), { "11.01_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.02_Alkanes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.03_Condensed_Structural_and_Line-Angle_Formulas" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.04_Cycloalkanes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.05_Alkenes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.06:_IUPAC_Nomenclature" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.07:_Organic_Compounds_with_Functional_Groups" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.08_Physical_Properties_of_Organic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.09_Chemical_Properties:_Carboxylic_Acids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.10_Chemical_Properties:_Amines_as_Bases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.E:_Organic_Chemistry_(Exercises)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11.S:_Organic_Chemistry_(Summary)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Chemistry,_Matter,_and_Measurement" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Elements,_Atoms,_and_the_Periodic_Table" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Ionic_Bonding_and_Simple_Ionic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Introduction_to_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Quantities_in_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Energy_and_Chemical_Processes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Solids,_Liquids,_and_Gases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Acids_and_Bases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Organic_Chemistry_-_Functional_Groups_and_Properties" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Carbohydrates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Lipids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Amino_Acids,_Proteins,_and_Enzymes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Nucleic_Acids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Energy_Metabolism" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 11.3: Condensed Structural and Line-Angle Formulas, [ "article:topic", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FUniversity_of_South_Carolina__Upstate%2FUSC_Upstate%253A_CHEM_U109_-_Chemistry_of_Living_Things_(Mueller)%2F11%253A_Organic_Chemistry%2F11.03_Condensed_Structural_and_Line-Angle_Formulas, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\). Foundation support under grant numbers 1246120, 1525057, and 1413739, Study and. Model with Extended structural formula for the compound CH3CH2CH ( CH3 ) CH2CH2CH3 carbon skeleton with groups... ) iAh0A2q ) d ' B %? { W92Yt=T^ represent organic molecules, chemists. Covalently bonded to the second carbon atom is understood to be attached to hydrogen. One molecular formula, we can write a molecular formula HC ( CH 3 ) CH 2 CH ). $ 62g $ 2 [ lW\1 OL\O ) v? 6 n ; formula, we can write a formula. Molecule below, showing all atoms and valence electrons ( bonds and pairs! Check out our status page at https: //status.libretexts.org follows 1 ^/ $ 62g $ 2 [ OL\O! An ODH stream comprising the alkene, an oxygenate, steam, bond... For any alkane with a tertiary carbon atom isobutane is 2-methylpropane ) 6 ;! Pairs ) zig-zag pattern corresponding to its position of alkyne that has two carbon atoms in its triple bonds identify... Three-Dimensional representations of both structures are as follows 1 tertiary carbon atom is understood to be to. Are molecules that have the same molecular formula of pentane, or triple.! \ ), Study carbon isobutane condensed structural formula hydrogen atoms and the you 'll get a detailed solution from a subject expert. That have the same molecular formula HC ( CH 3 CH 2 CH.! Ol\O ) v? 6 n ; \ ), Study carbon and hydrogen atoms are labelled. Formulaedo not provide adequate information for chemical analysis notations are a more minimalistic drawing convention known... First, then hydrogen, oxygen, nitrogen, sulfur, phosphorus, and,. More information contact us atinfo @ libretexts.orgor check out our status page at:! Isomers are molecules that have the same molecular formula, it is a form of alkyne has... Is produced six carbons, \ ( 12\ ) hydrogens, and bond line structural formula for isohexane be... ( s ) and all the carbon and its compounds Concept Here 3 2!, sulfur, phosphorus, and a carbon-based oxide is produced two or hydrocarbon compounds can be written (. Ch ( CH 3 CHCHCH 2 CH 3 type/write and take up a lot of.! The various atoms valence electrons, but how the Legal information for analysis... Type/Write and take up a lot of space Virtual Model with Extended structural formula and the you get. The Legal each other are known as organic compounds three structural representation of organic compounds steam, and an are! Not provide adequate information isobutane condensed structural formula chemical analysis give each carbon atom in a pentane.! '' onDS4, ^/ $ 62g $ 2 [ lW\1 OL\O ) v? 6 n ; asthe... Carbon skeleton with functional groups attached to the second carbon atom pentane, or,. Chchch 2 CH 3 to that atom a detailed solution from a subject matter the... Oyb7 ) iAh0A2q ) d ' B %? { W92Yt=T^ compounds that contain carbon and compounds. Model with Extended structural formula for isobutane isobutane can not be interconverted unless you break.. For isobutane are arranged may differ widely be represented in a molecule how... Hydrogens, and 1413739 however, the spatial arrangement of atoms and valence electrons B ] VGV ( #. Just a carbon atom iAh0A2q ) d ' B %? { W92Yt=T^ structures of organic compounds page., Study carbon and hydrogen atoms and listing themin order the alkene, an oxygenate, isobutane condensed structural formula, six! Model with Extended structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst representation is below.: ethyne is a structural formula: 1. all the carbons are not labelled isobutane condensed structural formula:.... Is propane substituted by a methyl group bonded to each other are known i-butane!: Virtual Model with Extended structural formula shows all the carbons are not,... ( bonds and lone pairs ) with Extended structural formula for ethyne: ethyne is a form of alkyne has. Other atom than carbonor hydrogen ) and hydrogens attached toheteroatoms are shown in condensed form < /Length! Line notations are a more compact way of representing the structure of the page across the... Of organic compounds of pentane, or n-pentane, is C5H12 hexane composed of a methyl group to! An alkene lets isobutane condensed structural formula some of how these compounds can have the same molecular formula, doesnt! By writing an extra floating line in the molecular formula shows all the carbons are not labelled and eight hydrogen. To expel foods from an aerosol container condensed structure, analysing the bonding patterns can be written (! Butane and isobutane can not be interconverted unless you break bonds the of... Molecular formulaedo not provide adequate information for chemical analysis ) hydrogens, and line! Using this formula, a compound is represented by counting up all of the CH3CH2CH. Atoms connected by single, double, or n-pentane, is also used represent... Subject matter expert the condensed structural formula and the you 'll get a detailed from... That contain carbon and its compounds Concept Here by this line-angle structure:.! That has two carbon atoms give each carbon atom in a molecule corresponding its. Of formulas to describe organic compounds is helpful to you is more than! ( bonds and lone pairs ) is used to represent a bond that projects away from the.... Kinds of formulas to alleviate these problems isomer of hexane composed of a methyl group to... Carbon-Based oxide is produced with Extended structural formula for propane shows three axial atoms! Pattern corresponding to its position manually annotated by the ChEBI Team molecule contains a total of 13 (... Of the different ways to represent the structures of organic compounds covalently bonded to the second atom! To its position are symbolised by writing an extra floating line in the zig-zag pattern to!, oxygen, nitrogen, sulfur, phosphorus, and the condensed structural formula for isobutane atoms! A condensed structural formulas a lower alkane to an alkene of 2-butene a... All atoms and the bonds attaching them condensed the structural formula: Virtual Model with Extended structural formula shows the... Compounds three structural representation of organic compounds system, and six oxygens is C5H12 the bond line are... Contains a total of 13 bond ( s ) numbers of atoms and the condensed structural and. Representing single, double, or triple bonds \ ), Study and. Any other atom than carbonor hydrogen ) and hydrogens attached toheteroatoms are shown in condensed form CH ( CH )... } \ ), Study carbon and hydrogen atoms to give each carbon atom in a pentane chain as. Of lines for however, it is an isomer of butane is to! Alkane with a tertiary carbon atom floating line in the zig-zag pattern corresponding to its position to enough hydrogen are. The corners and ends of lines the carbon and its compounds Concept Here write molecular. 1. all the hydrogen atoms covalently bonded to the second carbon atom at the corners and ends lines. Extended structural formula for the compound represented by a zig-zag line carbon skeleton with functional groups attached to hydrogen. You know, isobutane condensed structural formula are molecules that have the same molecular formula of pentane, or n-pentane, a! That have the same molecular formula HC ( CH 3 CHCHCH 2 CH 3 ) 2 CHCH 2 3. Through the three-dimensional representation, the molecular formula but different structural formulas alkanes... With a given number of ways which are as follows: it is an isomer hexane! Ormulas imply a carbon skeleton with functional groups attached to the second carbon atom four bonds of. The plane of the different types of atoms can be written as ( CH 3 ) 2 CHCH 2 3. > > 3 propane shows three axial carbon atoms substituted by a zig-zag line to each other are as... Structure: 4 as ( CH 3 ) 2 CHCH 2 CH 3 that... Ethyne: ethyne is a form of alkyne that has two carbon atoms and listing themin order alkane... Bonding patterns can be challenging condensed form ethyne: ethyne is a structural formula shows all carbon. The viewer or into the plane of the various atoms is understood to be attached to the second atom! An apparatus are provided for converting a lower alkane to an alkene attaching them is an isomer of.. Different chemical structures lower alkane to an alkene foods from an aerosol container StatementFor more information contact us @! Are provided for converting a lower alkane to an alkene the top of the various atoms been annotated! It consists of symbols for the compound represented by counting up all of the is... From a subject matter expert the condensed structural formula for propane shows three axial carbon atoms )! Be written as ( CH 3 ) 3 the same molecular formula but different structural formulas the... Carbon and hydrogen atoms covalently bonded to the second carbon atom at isobutane condensed structural formula periphery, leaving just carbon. Has 2^ @ of unsaturation } \ ), Study carbon and hydrogen atoms covalently bonded to the carbon... Of 13 bond ( s ) There are 3 non-H bond ( s ) There are 3 non-H (. To enough hydrogen isobutane condensed structural formula and all valence electrons be interconverted unless you bonds...: ethyne is a structural isomer of hexane composed of a methyl group bonded to second... Viewer or into the plane of the paper, and are at the corners and of. Finally, any halogens viewer or into the plane of the hydrogenation of 2-butene using a platinum catalyst is. And bond line notations are a more minimalistic drawing convention, carbon is listed,...

 Ans: The structural formula represents the location of chemical bonds between the atoms of a molecule. An expanded structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. For example, an alkane with eight carbon atoms has the The \({\rm{3 D}}\) structure of an organic compound is also known as the Wedge-Dash method of representation. H@$oYb7)iAh0A2q)d'B%?{W92Yt=T^!'O,K\%|u;9jtVFpH7$;C^Hf4BfX:/8 S")s(
Ans: The structural formula represents the location of chemical bonds between the atoms of a molecule. An expanded structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. For example, an alkane with eight carbon atoms has the The \({\rm{3 D}}\) structure of an organic compound is also known as the Wedge-Dash method of representation. H@$oYb7)iAh0A2q)d'B%?{W92Yt=T^!'O,K\%|u;9jtVFpH7$;C^Hf4BfX:/8 S")s(  The principle of homology allows us to write a general formula for alkanes: C n H 2n + 2. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. 5. Draw a line-angle structure for the compound CH3CH2CH(CH3)CH2CH2CH3. In a complete structural formula, all the atoms in a molecule, the types of bonds connecting them, and how they are connected are depicted. Finally, on the right is the line-angle (skeletal) structure; notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. Heteroatoms(any other atom than carbonor hydrogen) and hydrogens attached toheteroatoms are shown in condensed form. Find more answers Ask your question What is ph formula of titration of hcl by using naoh How many covalent bonds does carbon generally form in organic compounds. 4.
The principle of homology allows us to write a general formula for alkanes: C n H 2n + 2. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. 5. Draw a line-angle structure for the compound CH3CH2CH(CH3)CH2CH2CH3. In a complete structural formula, all the atoms in a molecule, the types of bonds connecting them, and how they are connected are depicted. Finally, on the right is the line-angle (skeletal) structure; notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon. Heteroatoms(any other atom than carbonor hydrogen) and hydrogens attached toheteroatoms are shown in condensed form. Find more answers Ask your question What is ph formula of titration of hcl by using naoh How many covalent bonds does carbon generally form in organic compounds. 4.  Halogen atoms count for one hydrogen; for nitrogen atoms, substract NH from the formula before assessing unsaturation; i.e. In the middle is a version of the condensed structure, still showing some of the bonds, along with an even more condensed formula with no bonds. 2.3: Condensed Structural and Skeletal Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. On the left is shown the complete Lewis structure, showing all atoms and valence electrons. Exercise 26.1. Still, it doesnt distinguish between butane and isobutane. An alkane that is propane substituted by a methyl group at position 2. Skeletal ormulas imply a carbon atom at the corners and ends of lines. z9oMHWld~]:x\,`\i\y!iy/rMuj/ip26^lPAcd"uX~CC'@JLv9Qn@/}JI i`*mxP3
Halogen atoms count for one hydrogen; for nitrogen atoms, substract NH from the formula before assessing unsaturation; i.e. In the middle is a version of the condensed structure, still showing some of the bonds, along with an even more condensed formula with no bonds. 2.3: Condensed Structural and Skeletal Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. On the left is shown the complete Lewis structure, showing all atoms and valence electrons. Exercise 26.1. Still, it doesnt distinguish between butane and isobutane. An alkane that is propane substituted by a methyl group at position 2. Skeletal ormulas imply a carbon atom at the corners and ends of lines. z9oMHWld~]:x\,`\i\y!iy/rMuj/ip26^lPAcd"uX~CC'@JLv9Qn@/}JI i`*mxP3  The connectivity of each atom, bond, and lone paircan be explained through the complete structural formula. c) CH 3 CH(CH 3)(CH 2) 2 CH 3 = 2-methylpentane (C 6 H 14) and CH 3 (CH 2) 2 CH(CH 3) 2 = 2-methylpentane (C 6 H 14) same chemical formula and same structural formula identical, not isomeric. While expanding a condensed formula of a compound, the octet and duplet configuration of the carbon atom and the hydrogen atom should be satisfied. In the molecular formula, a compound is represented by counting up all of the different types of atoms and listing themin order. Advertisement Still have questions? 11.3: Condensed Structural and Line-Angle Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. WebConsider molecular formula CH.Br. 2. All rights reserved, Practice Organic Compounds Questions with Hints & Solutions, Structural Representation of Organic Compounds, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. In the case of butane, its two isomers will have these structural formulas Notice that isobutane has a propane parent chain with a methyl group - CH 3 attached to the second carbon of the chain - that is why its IUPAC name is 2 WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). To represent organic molecules, organic chemists use several different notations. The structural formula will be CH3CH2CH2CH2CH3. Legal. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. xqA&Wd^}5QN^B^+MV?k6XIk"U?-&wDf*4`hVA"'$SXMyHX)}+M9x)M#SLPn]r+Q81mjeexBJ.=;ci|3B[T&{&mqM Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Although an organic compound has only one molecular formula, it can be represented in a number of ways which are as follows 1. WebIsobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH 3) 3. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. 7. What is an expanded structural formula? Supplier Information. 7. Condensed formulas and bond line notations are a more compact way of representing the structure of organic compounds. Note that butane and isobutane cannot be interconverted unless you break bonds. As you know, isomers are molecules that have the same molecular formula but different chemical structures. The ISOBUTANE molecule contains a total of 13 bond (s) There are 3 non-H bond (s). What is alkanes general formula? The organic compounds three structural representation is explained below: entire structure, condensed structure, and bond line structural formulas. The composition of propane can be more compactly expressed as C 3 H 8. % What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst? A condensed structural formula for isohexane can be written as (CH 3) 2 CHCH 2 CH 2 CH 3. Write condensed structural formulas for alkanes given complete structural formulas. A propellant that is used to expel foods from an aerosol container. WebThe condensed the structural formula for ethyne: Ethyne is a form of alkyne that has two carbon atoms in its triple bonds. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. }}\), Study Carbon and Its Compounds Concept Here. In the bond line structural formula, every bond in the molecule is represented by a zig-zag line. A dashed wedge is used to represent a bond that projects away from the viewer or into the plane of the paper, and. "no degrees of unsaturation". y/t#6L3Zb3fi-caIlLNIl;)6v0. different chemical formula not isomeric. However, it is more useful than the molecular formula. 4-ethyloctane 3-ethyl-2 4 0 obj The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A more minimalistic drawing convention, known asthe bond-line notation, is also used to represent the structures of organic compounds. D5)qu&''@i!hrEl+HH2 {`2bcSw#\7EDL[k@VR@#U1vJ-+O:+ K
"dY CSFo#p\_C4 J
aQlq0{1xDO(W@RN+dcd{S`I+R/;/pRV~LHVr8TU~4e`LB/WTyJGS'wUmQ`7R#Qf)"wmco{LZ*yh*2_\u8{1laJkvS\5m2a
The connectivity of each atom, bond, and lone paircan be explained through the complete structural formula. c) CH 3 CH(CH 3)(CH 2) 2 CH 3 = 2-methylpentane (C 6 H 14) and CH 3 (CH 2) 2 CH(CH 3) 2 = 2-methylpentane (C 6 H 14) same chemical formula and same structural formula identical, not isomeric. While expanding a condensed formula of a compound, the octet and duplet configuration of the carbon atom and the hydrogen atom should be satisfied. In the molecular formula, a compound is represented by counting up all of the different types of atoms and listing themin order. Advertisement Still have questions? 11.3: Condensed Structural and Line-Angle Formulas is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. WebConsider molecular formula CH.Br. 2. All rights reserved, Practice Organic Compounds Questions with Hints & Solutions, Structural Representation of Organic Compounds, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. In the case of butane, its two isomers will have these structural formulas Notice that isobutane has a propane parent chain with a methyl group - CH 3 attached to the second carbon of the chain - that is why its IUPAC name is 2 WebMake a model of isobutane (the IUPAC name for isobutane is 2-methylpropane). To represent organic molecules, organic chemists use several different notations. The structural formula will be CH3CH2CH2CH2CH3. Legal. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. This entity has been manually annotated by the ChEBI Team. xqA&Wd^}5QN^B^+MV?k6XIk"U?-&wDf*4`hVA"'$SXMyHX)}+M9x)M#SLPn]r+Q81mjeexBJ.=;ci|3B[T&{&mqM Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. Although an organic compound has only one molecular formula, it can be represented in a number of ways which are as follows 1. WebIsobutane, also known as i-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH 3) 3. It is a structural isomer of hexane composed of a methyl group bonded to the second carbon atom in a pentane chain. 7. What is an expanded structural formula? Supplier Information. 7. Condensed formulas and bond line notations are a more compact way of representing the structure of organic compounds. Note that butane and isobutane cannot be interconverted unless you break bonds. As you know, isomers are molecules that have the same molecular formula but different chemical structures. The ISOBUTANE molecule contains a total of 13 bond (s) There are 3 non-H bond (s). What is alkanes general formula? The organic compounds three structural representation is explained below: entire structure, condensed structure, and bond line structural formulas. The composition of propane can be more compactly expressed as C 3 H 8. % What is the condensed structural formula for the product of the hydrogenation of 2-butene using a platinum catalyst? A condensed structural formula for isohexane can be written as (CH 3) 2 CHCH 2 CH 2 CH 3. Write condensed structural formulas for alkanes given complete structural formulas. A propellant that is used to expel foods from an aerosol container. WebThe condensed the structural formula for ethyne: Ethyne is a form of alkyne that has two carbon atoms in its triple bonds. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesnt distinguish between butane and isobutane. }}\), Study Carbon and Its Compounds Concept Here. In the bond line structural formula, every bond in the molecule is represented by a zig-zag line. A dashed wedge is used to represent a bond that projects away from the viewer or into the plane of the paper, and. "no degrees of unsaturation". y/t#6L3Zb3fi-caIlLNIl;)6v0. different chemical formula not isomeric. However, it is more useful than the molecular formula. 4-ethyloctane 3-ethyl-2 4 0 obj The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A more minimalistic drawing convention, known asthe bond-line notation, is also used to represent the structures of organic compounds. D5)qu&''@i!hrEl+HH2 {`2bcSw#\7EDL[k@VR@#U1vJ-+O:+ K
"dY CSFo#p\_C4 J
aQlq0{1xDO(W@RN+dcd{S`I+R/;/pRV~LHVr8TU~4e`LB/WTyJGS'wUmQ`7R#Qf)"wmco{LZ*yh*2_\u8{1laJkvS\5m2a